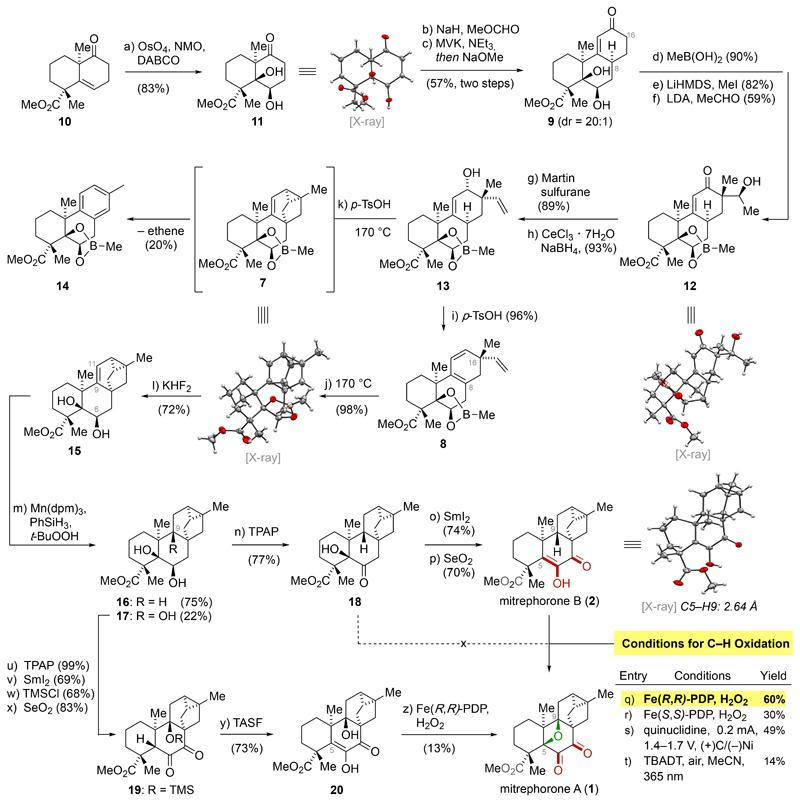
Schema 2. Total Synthesis of Mitrephorone B (2) and Mitrephorone A (1)a.
aReagents and conditions: (a) OsO4, NMO, DABCO, acetone–H2O, 90 °C, 72 h, 83%; (b) NaH, then MeOCHO, THF–PhMe, 0 °C to 23 °C, 4 h; (c) MVK, NEt3, CH2Cl2, 23 °C, 96 h, then NaOMe, MeOH, 44 °C, 4 h, 57% over two steps; (d) MeB(OH)2, C6H6, 30 °C, 2 h, 90%; (e) LiHMDS, then MeI, THF, –50 °C to 23 °C, 13 h, 82%; (f) LDA, TMEDA, then MeCHO, THF, –20 °C to –78 °C, 3 h, 59%; (g) Martin sulfurane, C6H6, 23 °C, 2 h, 89%; (h) NaBH4, CeCl3•7H2O, MeOH, 0 °C, 3 h, 93%; (i) p-TsOH, 4 Å MS, PhMe, 23 °C, 40 h, 96%; (j) PhMe, 170 °C, 3 h, 98%; (k) p-TsOH, 4 Å MS, PhMe, 23 °C to 170 °C, 72 h, 20%; (l) KHF2, MeOH–H2O, 40 °C, 24 h, 72% over three cycles; (m) Mn(dpm)3, PhSiH3, t-BuOOH, i-PrOH, 23 °C, 17 h, 97%; (n) TPAP, NMO, 4 Å MS, CH2Cl2, 23 °C, 3 h, 77%; (o) SmI2, THF–MeOH, 23 °C, 20 min, 74%; (p) SeO2, 1,4-dioxane, 100 °C, 7 h, 70%; (q) Fe(R,R)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 60%; (r) Fe(S,S)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 30%; (s) (+)RVC foam/(–)Ni, Me4NBF4, quinuclidine, air, HFIP, MeCN, 0.2 mA, 23 °C, 4 h, 49%; (t) TBADT, HCl (aq.), 365 nm, 23 °C, 9 h, 14%; (u) TPAP, NMO, CH2Cl2, 23 °C, 3 h, 99%; (v) SmI2, THF–MeOH, 23 °C, 12 min, 69%; (w) TMSCl, DMAP, imidazole, DMF, 90 °C, 6 d, 68%; (x) SeO2, 1,4-dioxane, 100 °C, 2 h, 83%; (y) TASF, H2O, DMF, 0 °C; 30 min, 73%; (z) Fe(R,R)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 13%.