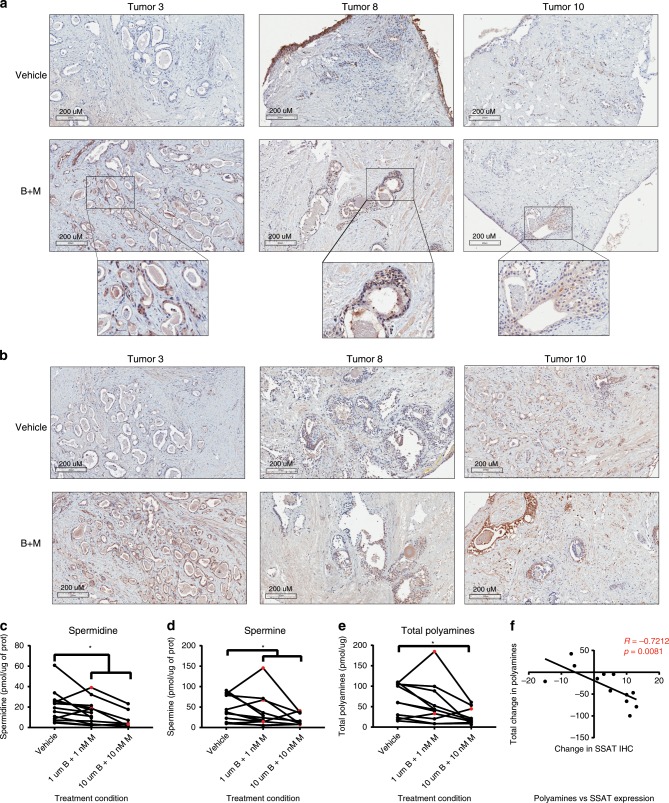
Fig. 6. BENSpm and MTDIA impact patient samples in ex vivo system.
Ex vivo samples were collected from treatment naïve patients undergoing radical prostatectomy at Roswell Park Comprehensive Cancer Center. Tumors used for this study had at least 40% neoplastic involvement upon pathological review. Tumor samples were treated with vehicle control (vehicle), or combination treatment (1 nM MTDIA + 1 µM BENSpm or 10 nM MTDIA + 10 µM BENSpm) for 7 days in the presence of 20 µM MTA. Images from representative tumors 3, 8, and 10 from immunohistochemical stained slides for a cleaved caspase-3 (CC3) or b spermidine/spermine N1-acetyltransferase (SSAT) in both vehicle and the combination treated samples (B + M. Intratumoral (c) spermidine (Vehicle; n = 13, 1 + 1 BM; n = 13, and 10 + 10 BM; n = 7), d spermine (Vehicle; n = 12, 1 + 1 BM; n = 12, and 10 + 10 BM; n = 9), and e spermidine + spermine (Vehicle; n = 11, 1 + 1 BM; n = 11, and 10 + 10 BM; n = 9) levels normalized to protein concentrations for vehicle and combination (B + M) treated tumor samples. f The correlation between the change in SSAT IHC Score and the change in total spermidine + spermine levels measured by UPLC in the combination treated samples (n = 12). Pearson’s r values are indicated. Statistical analysis for polyamines was done using a paired t-test. *p < 0.05.