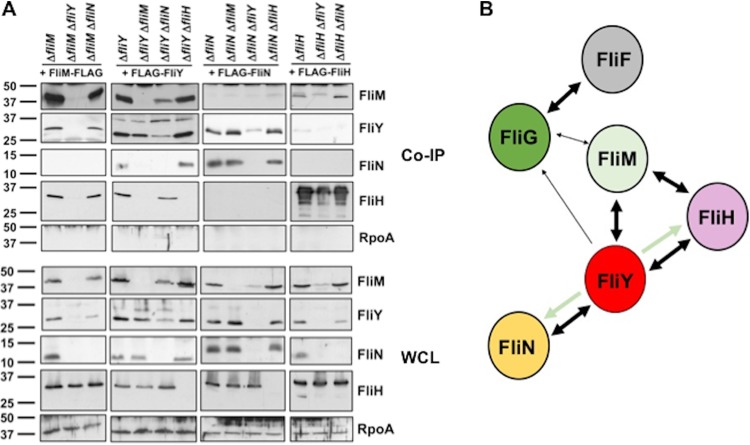
FIG 5.
Interdependencies of C-ring proteins on each other for interactions. (A) Coimmunoprecipitation (Co-IP) analysis of C-ring-protein interactions in mutants lacking another C-ring protein. C-terminal FLAG-tagged FliM or N-terminal FLAG-tagged FliY, FliN, or FliH was expressed from a plasmid in C. jejuni single or double mutants lacking specific flagellar proteins and immunoprecipitated by FLAG tag antibody resin after cross-linking cells by formaldehyde. Immunoprecipitated proteins were detected with specific antisera. The top set of immunoblots (Co-IP) shows results from coimmunoprecipitation experiments. The bottom set of immunoblots (WCL) shows levels of proteins in whole-cell lysates of mutants. RpoA is included as a control protein that should not coimmunoprecipitate with a C-ring protein and as a control to ensure equal loading of whole-cell lysates. (B) Summary model of C. jejuni C-ring-protein interactions from coimmunoprecipitation experiments. Double arrows indicate interactions verified by reciprocal coimmunoprecipitation experiments. Single arrows indicate an interaction that could only be observed by immunoprecipitation of one of two interacting partners. Thinner arrows indicate relative weaker interactions. Pale-green arrows indicate that the FliY interactions with FliH and FliN are dependent on FliM.