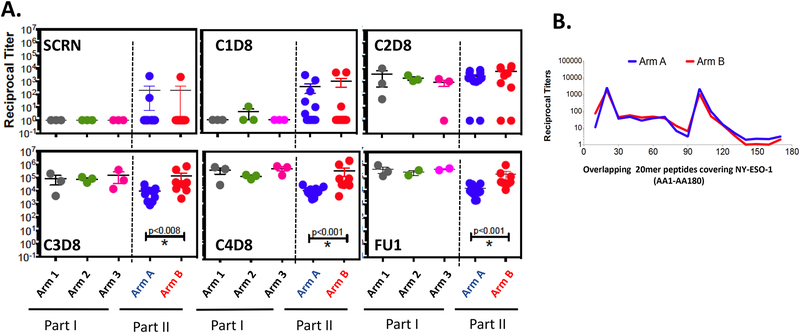
Figure 1:
Evolution of antibody titers against NY-ESO-1 by ELISA. A. Titer comparisons for NY-ESO-1 at screening and first follow-up (FU1) time points. B. Titer comparisons for NY-ESO-1 at different time points over the vaccination period. Arm 1 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1 mL montanide ISÄ−51 VG + 0.35 mg Poly-ICLC, Arm 2 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1mL montanide ISÄ−51 VG + 0.70 mg Poly-ICLC, Arm 3 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1mL montanide ISÄ−51 VG + 1.40 mg Poly-ICLC, Arm A (n=12): 100 μg NY-ESO-1 protein emulsified in 1.40 mg Poly-ICLC without montanide ISÄ−51 VG, Arm B (n=10): 100 μg NY-ESO-1 protein emulsified in 1.1 mL montanide ISÄ−51 VG + 1.40 mg Poly-ICLC. C. Seroreactivity to specific regions of the NY-ESO-1 protein were mapped using 20 mer overlapping peptides covering the entire protein.