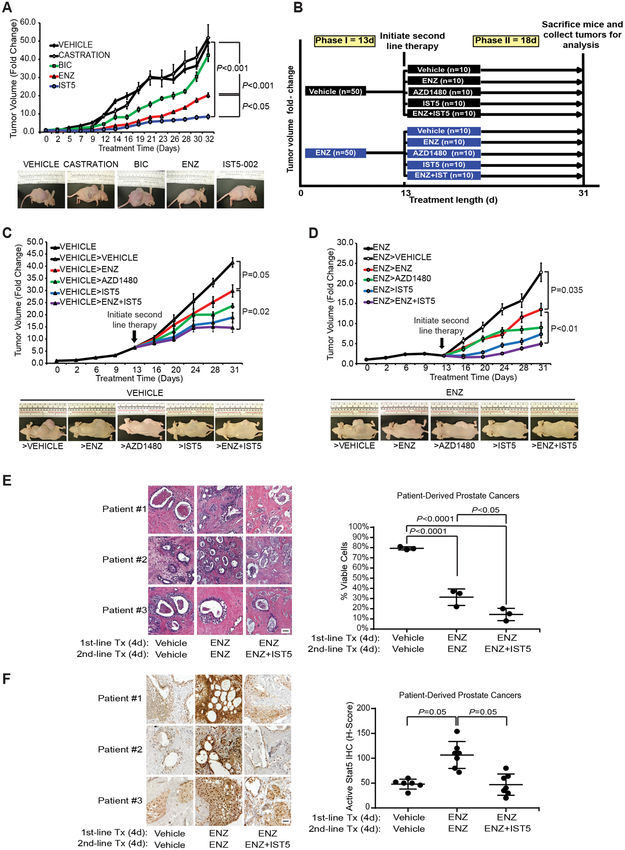
Figure 6. Second-line treatment by pharmacological inhibitors of Jak2-Stat5 signaling suppresses growth of both androgen-dependent and ENZ-resistant xenograft tumors in nude mice and in patient-derived prostate cancers ex vivo in tumor explant cultures.
A, Inhibition of Stat5 by IST5-002 suppressed androgen-sensitive CWR22Pc xenograft tumor growth more effectively than bicalutamide (BIC) or ENZ, as shown by tumor growth curves (upper panel) and representative tumor images (lower panel). CWR22Pc cells were inoculated subcutaneously (s.c.) into flanks of castrated athymic nude mice supplied with sustained-release DHT pellets. Mice were surgically castrated or treated daily with vehicle, BIC (30 mg/kg), ENZ (30 mg/kg) or IST5-002 (IST5) (50 mg/kg), and tumor growth rates were calculated for each treatment group and are presented as fold changes in tumor volume (volume at timepoint/volume at treatment start). B, Experimental design for the sequential therapy of the xenograft tumors in vivo. A two-phase in vivo experiment using vehicle or ENZ as first-line therapy (phase I, 13 days) and vehicle, ENZ, AZD1480, IST5-002 or ENZ+IST5-002 as second-line therapy (phase II, 18 days). On day 31, mice were sacrificed and CWR22Pc xenograft tumors collected for analyses. C-D, Sequential, second-line therapy with IST5-002 (IST5) or ENZ+IST5-002 (IST5) effectively suppressed the growth of androgen-sensitive (left panel) (C) and ENZ-resistant (right panel) (D). CWR22Pc xenograft tumors, as shown by tumor growth curves (upper panels) and representative tumor images (lower panels). CWR22Pc cells were inoculated s.c. into flanks of castrated athymic nude mice supplied with sustained release DHT pellets. Mice were treated with vehicle or ENZ (30 mg/kg) for 13 days. On day 13, mice were randomly distributed into subgroups and treated daily for an additional 18 days with vehicle, ENZ (30 mg/kg), AZD1480 (30 mg/kg), IST5-002 (IST5) (50 mg/kg) or ENZ (30 mg/kg)+IST5-002 (IST5) (50 mg/kg). Tumor growth rates are calculated and presented as described in (A). E, To test the efficacy of a second-line therapy with combined ENZ and IST5-002 (IST5) vs. ENZ monotherapy in clinical PCs, seven localized PCs obtained from radical prostatectomies were cultured ex vivo in tumor explant cultures in the presence of vehicle (8 days), ENZ (40 μM) alone (8 days) or ENZ (40 μM) as first-line therapy (4 days) followed by ENZ (40 μM) and IST5-002 (IST5) (25 μM) as a second-line therapy (4 days). Second-line therapy with combined ENZ and IST5-002 (IST5) induced cell death in clinical PCs more effectively than ENZ alone, as demonstrated by extensive loss of viable acinar epithelium. The number of viable epithelial cells in the explants at the end of the cultures were counted and presented as percentage per epithelial cells prior to culture. Representative histologies are shown (image magnification 40X; scale bar: 50μm). F, Levels of nuclear Stat5 in the PC explants were determined by immunostaining using biotin-streptavidin-amplified peroxidase-antiperoxidase immunodetection at the end of the cultures and expressed as percentages of Stat5 positive cells per 100 epithelial cells in explants for each treatment group (12-20 explants) (image magnification 40X; scale bar: 50μm).