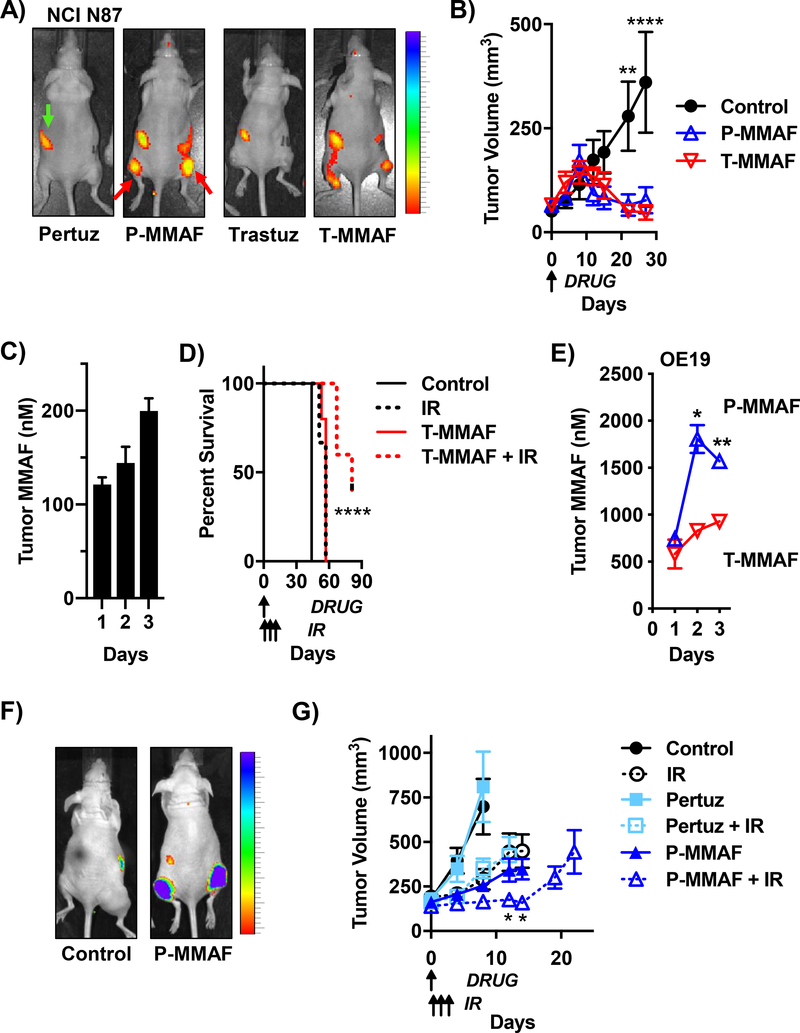
Figure 5: MMAF anti-HER2 antibody conjugates improve tumor control with IR.
A) Spatial localization of MMAF ADC in NCI N87 tumor xenografts grown in the thighs of mice (representative tumor locations indicated by red arrows in P-MMAF injected mouse). 1 nmole of unlabeled antibody (pertuzumab or trastuzumab) or Cy5 labeled P-MMAF or T-MMAF i.v. injected and whole mouse Cy5 fluorescence imaging 24 hours later. Gut auto-fluorescence indicated by green arrow. Scale bar on far right depicts dynamic range of Cy5 epi-fluorescence efficiency of 2.75×10−5 to 10×10−5. B) Anti-tumor efficacy of MMAF ADC in NCI N87 tumor-bearing mice. Mice i.v. injected on day 0 with 1 nmole P-MMAF or T-MMAF. Tumors measured twice a week and plotted as mean tumor volume ± SEM. Statistical significances calculated using two-way ANOVA with Tukey’s multiple comparisons test. C) Kinetics of ADC tumor drug accumulation in NCI N87 tumors. Mice i.v. injected with 1 nmole T-MMAF on day 0. Tumors harvested days 1, 2 and 3 post injection, drug concentrations quantitated and plotted as mean ± SEM. D) Anti-tumor efficacy of MMAF ADC with IR in NCI N87 tumors. Mice i.v. injected on day 0 with 0.5 nmole T-MMAF. 2.5 Gy given on days 1, 2 and 3. Mouse survival plotted and statistical significances calculated using Log-rank (Mantel-Cox) test. E) Anti-HER2 antibody drug delivery in OE19 tumors. Mice i.v. injected on day 0 with 1 nmole P-MMAF or T-MMAF. Tumors harvested days 1, 2 and 3 post injection, drug concentrations measured and plotted as mean ± SEM. Statistical significances calculated using unpaired t-test. F) Spatial localization of systemically delivered MMAF ADC in OE19 tumor xenografts. 1 nmole of P-MMAF i.v. injected and whole animal Cy5 fluorescence imaged 24 hours later. Scale bar on far right depicts dynamic range of Cy5 epi-fluorescence efficiency of 7×10−5 to 10×10−5. G) Anti-tumor efficacy of MMAF ADC with IR in OE19 tumors. Mice i.v. injected on day 0 with 0.5 nmoles pertuzumab or P-MMAF. IR given 2.5 Gy focally on days 1, 2 and 3. Tumors measured twice a week and plotted as mean tumor volume ± SEM. Statistical significances calculated using two-way ANOVA with Tukey’s multiple comparisons test. *P<0.05, **P<0.01, ****P<0.0001.