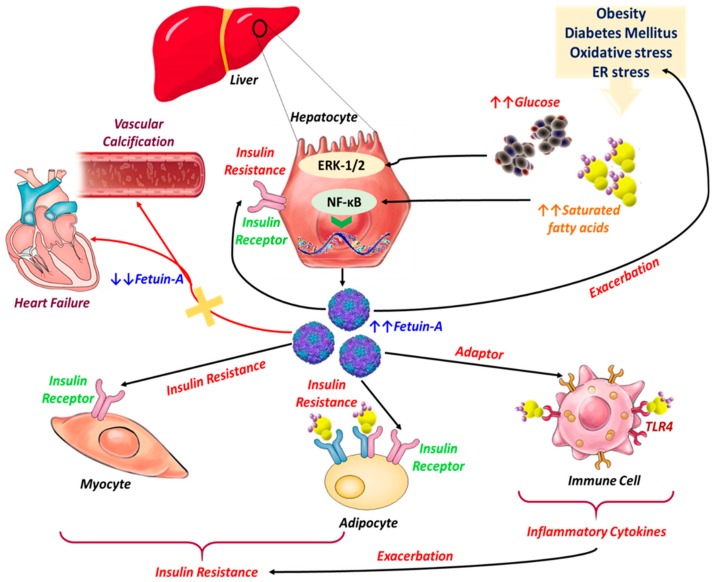
Figure 2.
Pathophysiological implications of Fet-A in the course of metabolic and cardiovascular diseases. Excessive release of Free fatty acids (FFAs) and overaccumulation of glucose in the bloodstream stimulate the biosynthesis of Fetuin-A from hepatocytes through activation of both Nuclear factor kappa B (NF-κB) and ERK 1/2 pathways. Fet-A acts then as an insulin signaling pathway inhibitor by modulating the kinase reaction initiated by insulin on insulin-receptor tyrosine kinase autophosphorylation; insulin-sensitive tissues become less responsive to insulin, triggering insulin resistance. Meanwhile, Fet-A functions as an adaptor between FFA and Toll like receptor 4 (TLR4) signaling in lipid-induced inflammation, and TLR4 signaling leads to the activation of NF-κB and Activator protein 1 (AP-1), which can then upregulate the transcription of inflammatory genes, resulting in the production of inflammatory cytokines that can lead to insulin resistance. On the other hand, decreased Fet-A synthesis is strongly correlated with excessive vascular calcification and general heart failure, as the Fet-A-stabilization effect toward insoluble calcium and phosphate crystals is no longer properly achieved.