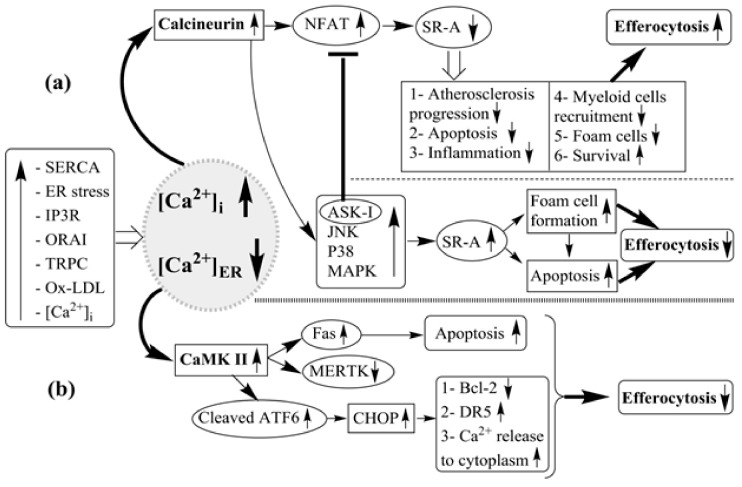
Figure 3.
Molecular pathways linking endoplasmic reticulum stress and subcellular Ca2+ movements to efferocytosis in atherosclerosis: Effects of upregulation of sarcoplasmic reticulum Ca2+-ATPase (SERCA), endoplasmic reticulum (ER) stress, inositol 1,4,5-trisphosphate receptor (IP3R), ORAI, transient receptor potential canonical (TRPC), oxidized low density lipoprotein (Ox-LDL), and [Ca2+]i on the calcineurin-mediated and calmodulin kinase II (CaMKII)-mediated cellular pathways associated enhanced or diminished efferocytosis. (a) The Orai1-activated increase of [Ca2+]i stimulates calcineurin, which results in triggering of two signaling pathways including calcineurin–apoptosis signal-regulating kinase 1 (ASK1)-p38/JNK and calcineurin-nuclear factor of activated T cells (NFAT) [108]. Mitogen-activated protein kinases (MAPKs), c-Jun N-terminal kinase (JNK), and p38 MAP have critical roles in the modulation of class A scavenger receptors (SR-A) expression [108]. The NFAT transcription factors act as negative modulators of SR-A expression [109]. A signaling pathway, which positively modulates the SR-A expression and stimulates foam cell formation in vitro, is calcineurin–ASK1-p38/JNK signaling pathway, while the calcineurin–NFAT pathway is a negative regulator of SR-A expression [109]. Activation of ASK1 via a negative feedback mechanism restricts the inhibitory influence of NFAT on the expression of SR-A [109]. (b) The released Ca2+ triggers CaMKII, which leads to activation of apoptotic pathways. Especially, CaMKII has many roles including the signal transducer and activator of transcription 1 (STAT1) activation, triggering the proapoptotic signal transducer and stimulation of the FAS death receptor by JNK [71,105]. ER stress leads to an increase in [Ca2+]Cyt, which in turn prompts FAS death receptor expression via the CaMKIIγ and JNK pathways in vivo [71]. CaMKII knockdown enhances macrophage efferocytosis receptor tyrosine-protein kinase MER (MerTK) expression, which in turn increases efferocytosis, and, in atherosclerotic lesions, should ultimately result in smaller necrotic cores. The increase of ATF6 leads to an enhanced efferocytosis through the expression of MerTK and in the absence of CaMKIIγ in macrophages [110]. Cleavage of ATF6 leads to an increase of C/EBPα-homolog protein (CHOP). Increase in the CHOP promotes cytosolic Ca2+ in the macrophages via CHOP-mediated stimulation of oxidase ERO1α in the ER, which in turn triggers Ca2+ release from the IP3R channel in the membranes of ER and also in an increased expression of the death receptor-5 (DR5) and in decreased expression of the death receptor-5 (Bcl-2), a mediator of cell survival [104,111]. These alterations result in increased cellular apoptosis and in reduced efferocytosis, a combination which is particularly pro-atherogenic.