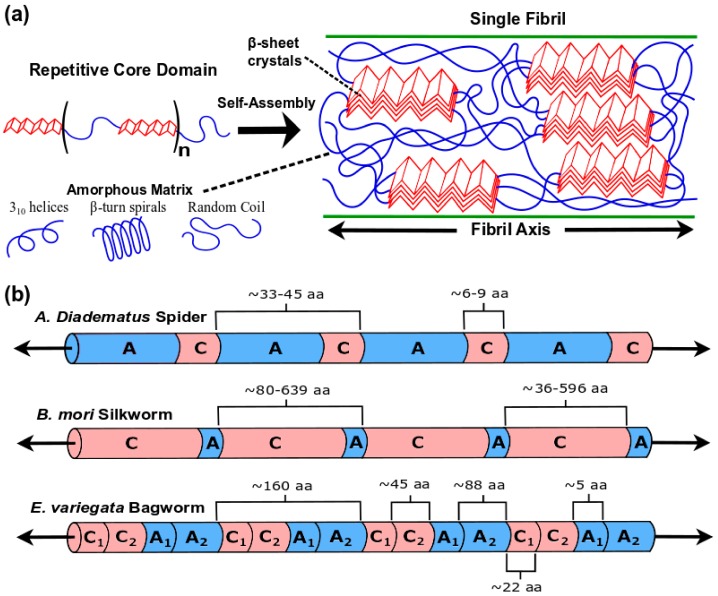
Figure 3.
(a) Schematic of silk fibroin primary sequence, showing a repetitive core domain consisting of alternating rigid and flexible blocks. Self-assembly of silk fibroin results in the formation of nanocrystals, which consist of “stacked” β-sheets with hydrophobic interactions between amino acid side chains extending orthogonal from the sheet plane, embedded in a hydrophilic amorphous matrix. (b) Silk fibroin from different species exhibit varying primary sequences, with corresponding differences in mechanical properties. A. diadematus ADF-4 dragline spidroin consists of alternating flexible “amorphous” (A) and rigid “crystalline” (C) blocks, where C is 6–9 alanines and the A-C length is typically 33–45 amino acids (aa) long. B. mori silk fibroin consists of A-C repeats ranging from 80–639 amino acids where C is rich in glycine-X motifs. E. variegata silk fibroin consists of A-C repeats approximately 160 amino acids long, where C and A blocks are divided into 2 distinct sections. C1 specifically consists of a (A)9E(A)12 sequence, while C2 and A2 are glycine-rich. A1 is a short linker rich in valine.