Abstract
In addition to their cholesterol-lowering effects, statins are associated with pleiotropic effects including improvements in heart failure (HF), reduced blood pressure, prevention of the rupture of atherosclerotic plaques and improved angiogenesis. In addition to these cardiovascular benefits, statins have been implicated in the treatment of neurological injuries, cancer, sepsis, and cirrhosis. These cholesterol-independent beneficial effects of statins are predominantly mediated through signaling pathways leading to increased production and bioavailability of nitric oxide (NO). In this review, the mechanistic pathways and therapeutic effects of statin-mediated elevations of NO are described and discussed.
Keywords: statin, endothelial nitric oxide, angiogenesis, heart failure, atherosclerosis, cancer, neuroprotection
1. Introduction
Statins reduce the endogenous synthesis of cholesterol by inhibiting hepatic hydroxymethylglutaryl (HMG) CoA reductase, the rate-limiting step of the mevalonate pathway of cholesterol production. This causes an upregulation of hepatic low-density lipoprotein (LDL) receptors and decreased deposition of LDL particles in the vascular walls, a necessary event in the pathogenesis of atherosclerosis. Pleiotropic effects of statins make this drug class distinct from other lipid-modifying agents [1,2] are those effects independent of cholesterol-lowering. Numerous putative therapeutic benefits have been ascribed to statins including suppression of apoptosis, antioxidant and anti-inflammatory effects, immunomodulation, neuroprotection and promotion of tissue regeneration [3,4,5,6,7,8,9]. Several of these effects are mediated through the actions of statins on endothelial nitric oxide (NO) signaling pathways.
Several studies have confirmed that statins upregulate the production and activity of NO [10,11]. Stains including lovastatin [12], simvastatin [13], atorvastatin [14], rosuvastatin [15], fluvastatin [16], pravastatin [17], and cerivastatin [16], increase NO through a variety of mechanisms and pathways, which increase the expression and function of endothelial NO synthase (eNOS). For example, 14 days of exposure of mice to atorvastatin has been shown to evoke an approximately three-fold increase in the expression and activity of eNOS [14]. Statins also increase bioavailable NO by preventing the elimination of NO by reactive oxygen species (ROS) [18,19].
In this review, we will discuss the molecular mechanisms and signaling pathways by which statins can modulate NO, and thereby exert cholesterol-independent pleiotropic therapeutic effects.
2. Signaling Pathways and Molecules Mediating the Effect of Statins on NO Production
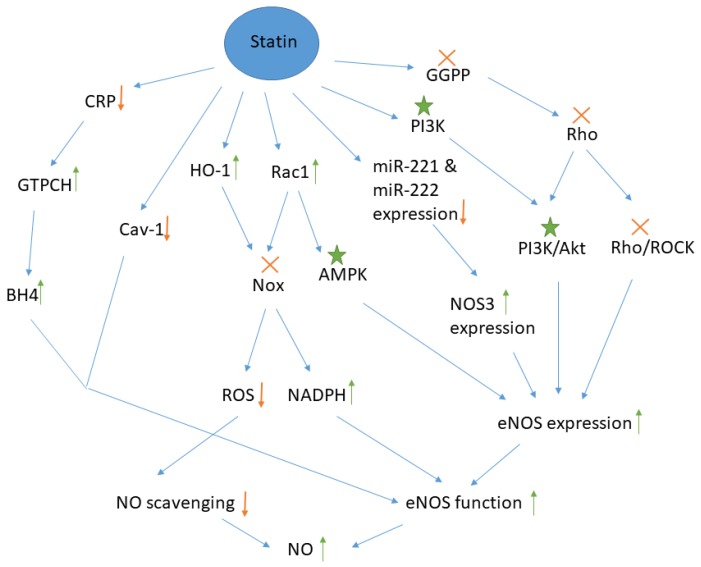
Statins can influence many signaling pathways and molecules which regulate the production of NO by endothelial cells. The major pathways are summarized schematically in Figure 1. Statins increase the expression of eNOS, mainly through inhibition of the Rho/ROCK pathway or by activation of either the Phosphoinositide 3-kinase (PI3k)/ Protein kinase B (Akt) or the AMP-activated protein kinase (AMPK) pathways. Inhibition of mevalonate synthesis by atorvastatin, simvastatin and lovastatin causes upregulation of Rho GTPase expression in the cytosol. The result of Rho GTPase upregulation is inactivation of Rho, because statins make isoprenoids such as geranylgeranyl pyrophosphate (GGPP) unavailable for isoprenylation and post-translational modification of Rho protein, thereby interfering with translocation of this protein from cytosol to the membrane and its activation. Inactive Rho inhibits the Rho/Rho-kinase (ROCK) pathway, which leads to an increase in the half-life of eNOS mRNA and increased production of NO [13,14,20].
Figure 1.
Molecules and pathways involved in the effect of statins on NO production by endothelial cells.  : Molecule has become unavailable or inactivate or the signaling pathway is suppressed,
: Molecule has become unavailable or inactivate or the signaling pathway is suppressed,  : Increase or decrease in the amount,
: Increase or decrease in the amount,  : Molecule is activated. GGPP: geranylgeranyl pyrophosphate, ROCK: Rho-associated protein kinase, PI3k: Phosphoinositide 3-kinase, Akt: Protein kinase B, AMPK: AMP-activated protein kinase, miR: micro RNA, HO-1: Heme oxygenase 1, BH4: tetrahydrobiopterin, Nox: NADPH oxidase, Cav-1: caveolin-1, GTPCH: guanosine-5-triphosphate cyclohydrolase, CRP: C reactive protein, NO: nitric oxide, eNOS: endothelial NO synthase, ROS: reactive oxygen species. It is noteworthy that Rac1 is actually a downstream signal for GGPP and the statin-mediated inactivation of GGPP reduces Rac1 level but as statins exert a direct inductive effect on Rac1, the up-regulation of Rac1 and its downstream signaling are shown separately here.
: Molecule is activated. GGPP: geranylgeranyl pyrophosphate, ROCK: Rho-associated protein kinase, PI3k: Phosphoinositide 3-kinase, Akt: Protein kinase B, AMPK: AMP-activated protein kinase, miR: micro RNA, HO-1: Heme oxygenase 1, BH4: tetrahydrobiopterin, Nox: NADPH oxidase, Cav-1: caveolin-1, GTPCH: guanosine-5-triphosphate cyclohydrolase, CRP: C reactive protein, NO: nitric oxide, eNOS: endothelial NO synthase, ROS: reactive oxygen species. It is noteworthy that Rac1 is actually a downstream signal for GGPP and the statin-mediated inactivation of GGPP reduces Rac1 level but as statins exert a direct inductive effect on Rac1, the up-regulation of Rac1 and its downstream signaling are shown separately here.
The mechanism of PI3K/AKT activation by statins is not fully understood, but it is known that PI3K phosphorylates, and thereby activates Akt [21]. Phosphorylated Akt stimulates phosphorylation of eNOS and NO production in cultured endothelial cells and endothelial progenitor cells (EPC) [22,23,24]. PI3K inhibitors suppress the effect of statins on Akt, demonstrating that the PI3K/Akt pathway is involved in the effect of statins on NO production [25]. Furthermore, Rho inhibition by statins promotes activation of Akt and conversely, overexpression of Rho leads to inhibition of eNOS activation [13,26]. The mediator of eNOS phosphorylation in the PI3K/Akt pathway is thought to be heat shock protein 90. Tyrosine phosphorylation of this protein by statins facilitates its direct interaction with Akt and drives the formation of an Akt/eNOS complex, which results in eNOS activation [27].
Activation of the AMPK pathway is another mechanism by which statins may enhance NO production. For example, incubation of human umbilical vein endothelial cells (HUVECs) with atorvastatin increased AMPK activity and eNOS phosphorylation in a time and dose-dependent manner [28]. AMPK enzymatically inactivates enzymes involved in cholesterol biosynthesis such as HMG-CoA. Therefore, statins and other inhibitors of HMG-CoA activate AMPK [17,29,30,31,32,33]. Based on in vitro and in vivo studies, atorvastatin, pravastatin, and simvastatin increase AMPK activation via phosphorylation at Thr-172 by increasing the GTP-bound Rac1 which promotes eNOS phosphorylation at Ser-1177. As expected, AMPK inhibitors block the effect of simvastatin on eNOS phosphorylation [17]. Interestingly, activation of AMPK by statins is dependent on the duration of exposure and whilst statin treatment increases AMPK activity after four hours, AMPK activity returns to baseline after 24 h [28].
Increasing the expression of NOS3, a gene encoding eNOS is another mechanism by which statins increase the generation of NO in endothelial cells. However, bioinformatic studies have not identified the target region for these microRNAs on the NOS3 mRNA molecule. Cerda et al. showed that microRNAs such as miR-221 and miR-222 reduce the expression of NOS3. Simvastatin reduces expression of miR-221 and atorvastatin mediates expression of both miR-221 and miR-222 in cultured HUVECs, thereby increasing NOS3 gene expression and eNOS function [34]. NO production could be altered through epigenetic modifications exerted by statins. These drugs change the expression of miRNAs and their corresponding target pathways, namely the Rho pathway, thereby regulating NO release from the endothelial cells [35]. Antioxidant properties are a well-recognized pleiotropic effect of statins [36,37]. The signaling molecules responsible for this effect are also responsible for the effects of statins on NO. Statins inhibit superoxide formation by blocking the activity of the oxidative stress-producing enzyme, NADPH oxidase (Nox), by facilitating membrane translocation of Rac1, a cytosolic subunit of Nox [38]. Wassmann et al. showed that atorvastatin reduced expression of essential membrane-bound subunits of Nox, p22phox, and Nox1 [39]. Simvastatin has also been shown to inhibit expression of Nox1 and Nox2 genes in osteocytes [40]. Both Rac1 translocation and heme oxygenase 1 (HO-1) upregulation by statins affect Nox inactivation. The enzyme HO-1 is an intracellular target for statins, and its upregulation reduces iNOS expression, O2- generation, and Nox availability. Finally, Nox inactivation leads to higher NO bioavailability by two mechanisms. Firstly, Nox inactivation prevent ROS production and NO scavenging by free radicals [18,19], and secondly, lack of Nox activity increases the amount of available NADPH, a cofactor for the synthesis of NO by eNOS from L-arginine in the presence of O2 [41].
Statins increase the concentration of tetrahydrobiopterin (BH4) by two mechanisms. BH4 is a cofactor for eNOS function, and hence increased BH4 may explain increased availability of NO. Statins can increase availability of BH4 by increasing the activity of GTP cyclohydrolase 1 (GTPCH), a rate-limiting enzyme in the synthesis of BH4. This appears to be achieved via a statin-induced reduction in C reactive protein (CRP), a suppressor of GTPCH [42]. Additionally, statins prevent the oxidation of BH4 to BH3. The result of these processes is reduced oxidative stress in endothelial cells and improved eNOS function [43].
One proposed mechanism for the effect of statins on NO, which is still controversial, is the inhibition of eNOS inactivation by caveolin-1 (Cav-1) following statin treatment [44]. Caveolae are distinct regions on the plasma membrane containing cholesterol and caveolin proteins which mediate cell signaling [45,46]. Cav-1 from caveolae inactivates eNOS through its attachment via N-terminal myristoylation and palmitoylation [47]. Cholesterol in the caveolae affects the Cav-1 function so that the exposure of cells to free cholesterol results in up-regulation of Cav-1 expression [48]. Therefore, statins play a role in the Cav-1 downregulation by inhibiting biosynthesis of cholesterol [49]. As a result, eNOS/Cav-1 complexes cannot be formed, and eNOS remains in its active state. Meda et al. transfected HUVECs with Cav-1 siRNA using magnetofection method, to decrease Cav-1 production. Then, NOS activity in the control cell and transfected cells and their subcellular fractions was measured after exposure of cells to statins such as fluvastatin, lovastatin, and cerivastatin. The results showed that these statins increased eNOS activity and NO synthesis in a concentration-dependent manner, which was evidenced by the increased accumulation of intracellular cGMP as an indicator of NO bioactivity. In this study, statins did not alter Cav-1 expression and it was hypothesized that statins instead altered the translocation of Cav-1 [16].
All the pathways and signaling molecules by which statins may elevate NO additionally activate other signaling pathways such as the stromal cell-derived factor 1 α (SDF-1α)/ CXC chemokine receptor-4 (CXCR-4) pathway. It has been shown that a seven-day infusion of atorvastatin in rats suffering from acute myocardial infarction increased eNOS and eNOS phosphorylation, activated the eNOS/NO pathway, and finally increased expression of CXCR4 and SDF-1α [50]. Activation of the SDF-1α/ CXCR-4 pathway promotes EPCs mobilization and differentiation into vessel-forming cells [51].
3. Therapeutic Implications of Statin-Induced Elevation of NO
The statin-induced elevation of NO described above has important clinical implications. NO is an important mediator of vasodilatation, it prevents leukocyte adhesion, smooth muscle cell proliferation, and platelet aggregation, and it improves endothelial and vascular function [52]. Improving endothelial function by statins suggests their potential therapeutic effects in cardiovascular diseases such as atherosclerosis [53], hypertension [54,55], and HF [56]. Enhanced NO production by endothelium could also provide protection against ischemia in the brain [57]. Whether the effect of NO is to promote new vessel formation in ischemia or to block angiogenesis in tumors and atherosclerosis plaques may depend upon its local concentration in the tissues of interest [58].
3.1. Heart Failure
It has been reported that simvastatin increases eNOS expression and NOS activity in the left ventricle (LV) and aorta of rats [56]. In animal models of chronic heart failure (CHF), treatment with cerivastatin or fluvastatin after myocardial infarction (MI) resulted in better LV function and survival [59,60]. In a randomized controlled trial, oral simvastatin improved myocardial ultrastructure [61]. In CHF patients, flow-mediated dilatation was enhanced after the treatment of patients with rosuvastatin [62]. Some studies have shown that atorvastatin has cardioprotective effects, improves echocardiographic parameters, prevents LV dilatation, and delays the progression of HF. The improved myocardial remodeling by atorvastatin was attributed to reduced CRP and inflammation, and increased angiogenesis in cardiac muscle fibers. These effects are mediated by NO production [63]. Atorvastatin blocks the RhoA/Rho kinase pathway and increases the amount of mRNA for eNOS [64]. Upregulation of eNOS expression by statins increases capillary density and/or improves endothelial function, leading to better myocardial perfusion and improvement of HF [65]. The beneficial effects of statins on cerebral ischemia were not evident in eNOS gene knockout mice, confirming that these effects are mediated by NO [66]. However, there are controversies regarding beneficial effects of statins in HF [67,68,69].
3.2. Hypertension
A meta-analysis of randomized controlled trials of statin therapy has shown that statins, including pravastatin and simvastatin reduce systolic blood pressure by about 2-mmHg in both hypertensive and normotensive patients [54,55].
Due to the immediate effects of statin on blood pressure and a lack of relationship with the cholesterol response demonstrated in meta-analysis, the mechanism of this effect has been attributed to increase NO production by endothelial cells, and decreased concentrations of endothelin-1, a potent vasoconstrictor protein [54,70]. NO is a potent endogenous vasodilator which causes vasodilatation through binding the soluble guanylyl cyclase, the enzyme responsible for the conversion of guanosine-5-triphosphate (GTP) to cyclic 3,5-guanosine monophosphate (cGMP) [52]. Additionally, the vasodilator effect of statins results from prevention of the generation of ROS, including hydroxyl radicals [71]. Furthermore, statins decrease arterial stiffness and improve systemic arterial compliance by altering the smooth muscle cells composition in artery walls and by restoring endothelial function [71,72]. Studies have demonstrated that incubating isolated rat mesenteric resistance arteries with simvastatin reduces contractility, increases vessel diameter and results in hypotension in such arteries via NO-dependent mechanisms [17,73].
3.3. Atherosclerosis
The pivotal role of NO in atherosclerosis process and the NO elevating effect of statins mean that statins have the potential to modulate the course of this condition independently from their well-characterized lipid-lowering effects. Endothelial dysfunction is an early manifestation of atherosclerosis. In the physiological state, the release of NO from endothelial cells protects the endothelium from platelet adhesion in addition to its vasodilator effects. In the early stages of atherosclerosis, oxidative stress, characterized by impairing NO bioavailability results in endothelial dysfunction. Increased oxidative stress at atherosclerotic site turn O2 to ROS such as O2- which readily reacts with NO to form peroxynitrite (ONOO-) or nitrosothiols. ONOO−is a highly reactive, highly toxic, and pro-inflammatory agent that oxidizes and inactivates eNOS [74,75].
Statins have the potential to slow the progression of atherosclerosis through NO-mediated mechanisms, and conversely, the rate of development of atherosclerosis in Apolipoprotein E (ApoE) mice is increased when they lack eNOS [53]. In vitro treatment of injured HUVEC cells as a model of dysfunctional vascular endothelial cells in atherosclerosis showed that rosuvastatin reduced cell apoptosis by increasing NO content [15].
Two distinct mechanisms govern the beneficial effects of statins on atherosclerosis. Firstly, statins activate AMPK, which inhibits Nox phosphorylation and activation by protein kinase C (PKC) [76,77]. The antioxidant properties of statins prevent ROS generation and the formation of ONOO−. For example, simvastatin with L-arginine supplementation reduced ONOO− in hypercholesterolemic subjects [78], thereby potentially preserving eNOS activity. Secondly, statins improve stability of the plaques, thereby making them less prone to rupture. Although the mechanism for this effect is yet unclear, it has been hypothesized that statins induce plaque calcification [79,80]. By restoring the NO/ONOO− balance and preventing the progression of atherosclerosis, statins activate eNOS, increase NO bioavailability, reduce the release of ONOO− from the endothelium, and increase HO-1 expression and total HO activity [81].
A study which included 30 patients with peripheral arterial diseases showed that one-month of treatment with atorvastatin significantly reduced plasma ONOO− levels and reduced oxidative stress, resulting in reduced eNOS uncoupling and increased NO production [43]. Rasmusen et al. also showed that atorvastatin combined with a NO precursor, arginine, exert anti-atherosclerotic actions via eNOS activation and NO production [82].
3.4. Sepsis
Sepsis is an inflammatory disease consisting of pathological changes in arteriolar tone and endothelial cell integrity, leading to severe hypotension. Loss of NO, overproduction of inducible nitric oxide synthase (iNOS), and the imbalance of iNOS and eNOS are responsible for this disease. Statins such as cerivastatin, simvastatin, atorvastatin, fluvastatin, and pravastatin, increased survival in experimental sepsis models by restoring the iNOS/eNOS balance. In sepsis treatment, hydrophilic statins have priority over hydrophilic ones due to their higher lifetime and potency [83].
3.5. Cirrhosis
Statins have beneficial effects on hepatic disorders such as cirrhosis. In cirrhosis, intrahepatic vascular resistance increases due to endothelial dysfunction caused by impaired NO bioavailability. Simvastatin and atorvastatin have been shown to reduce ROS generation, increase eNOS activity and preserve NO availability. The resultant vasodilation leads to a reduction of portal pressure selectively in the hepatic microvasculature and a reduction of hepatic resistance. These effects have been demonstrated in an in vivo rat model of cirrhosis and confirmed in clinical trials [84,85,86,87].
3.6. Therapeutic Induction of Angiogenesis and Vasculogenesis
Therapeutic angiogenesis and vasculogenesis are the generation of new blood vessels in conditions resulting from insufficient vasculature, such as ischemic tissues or tissue engineering scaffolds [88]. Statins induce therapeutic angiogenesis and vasculogenesis (Figure 2). For example, rosuvastatin treatment causes circulating EPCs originating from bone marrow to mobilise into the ischemic site of a mouse model of surgically induced hindlimb ischemia, through the activation of Akt/eNOS pathway [89]. A study by Urbich et al. demonstrated that low doses of atorvastatin and mevastatin (0.01 to 0.1 µmol/L) improve the migration of mature endothelial cells and improve tube formation and angiogenesis. These statins also increased vasculogenesis through actions on circulating EPCs. The involvement of both mature endothelial cells and circulating EPCs after statin treatment suggests that statins can induce both angiogenesis and vasculogenesis [89,90,91]. Therefore, statins have been loaded into tissue engineering scaffolds made from mesoporous hydroxyapatite microspheres or titanium to induce angiogenesis in the structure [92,93].
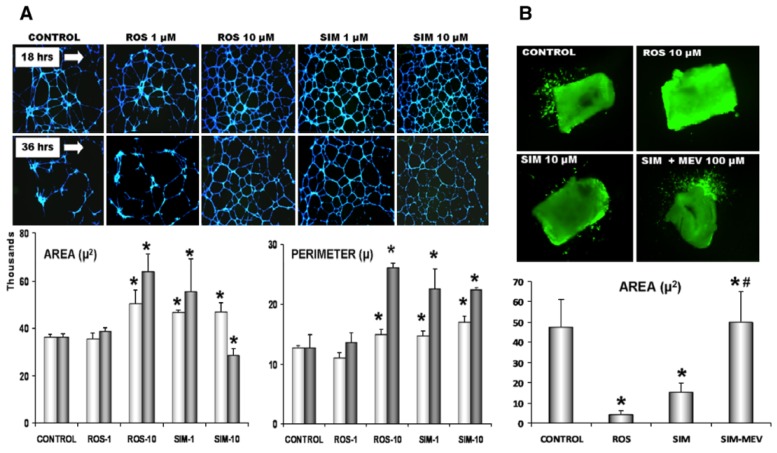
Figure 2.
Induction of angiogenesis and vasculogenesis by statins. (A) Matrigel angiogenesis assay. HUVECs were exposed to rosuvastatin (Ros) and simvastatin (SIM) at 1 and 10 μM for 18 (upper panel of images) and 36 h (lower panel). Representative images show cells loaded with calcein for fluorescent analysis: Gr *p < 0.05 vs. untreated control. (B) Representative images showing the effects of ROS pretreatment with 100 µM L-mevalonate on simvastatin-treated rings; the graph displays simvastatin-treated aortic rings. Data is shown as means ± SE, based on 3–4 independent experiments. Reproduced with permission from [94].
The ability of cells to form tubes after statin exposure is mediated by both the PI3K/Akt pathway (evidenced by suppression of migration by a PI3k inhibitor) and AMPK pathways. Sun et al. showed that the effect of atorvastatin on angiogenesis and tube formation ability of HUVECs is mediated by AMPK activation [28]. Furthermore, statins activate endothelial Ras which activates Akt phosphorylation. Activation of Akt in this pathway leads to posttranscriptional activation of the eNOS. Increased eNOS phosphorylation leads to eNOS/NO pathway activation and NO production. For example, exposure of transplanted mesenchymal stem cells (MSCs) to atorvastatin under hypoxic conditions increased neovascularization in peri-infarcted areas of the heart by upregulating eNOS [95,96]. In another experiment, loading statin into a tissue engineering scaffold designed for regenerating intractable diabetic skin wounds promoted angiogenesis through upregulation of eNOS and NO synthesis [97].
3.7. Neuroprotection
Neuroprotection by statins occurs through a variety of mechanisms including reduced expression of the mammalian target of rapamycin (mTOR) protein, increasing brain-derived neurotrophic factor (BDNF) and glial-cell-line-derived neurotrophic factor (GDNF) [98]. Generation of NO by eNOS and nNOS (neuronal NOS) is another mechanism of neuroprotection. NO regulates cerebral blood flow after brain injuries and is a potent neuroprotective factor [57]. The mechanism of cerebral blood flow regulation by eNOS is shown in Figure 3. Therefore, statins are beneficial in the treatment of brain ischemia because they increase the expression of eNOS by inhibiting changes in Rho-mediated actin cytoskeleton [99]. Expression of eNOS is decreased in some neurological injuries, such as strokes and cerebral artery occlusion [57]. In these situations, statins exert neuroprotective effects through restoring eNOS expression. Cerebral blood flow is enhanced by eNOS, stroke severity is reduced and neurological function is improved, as demonstrated by the fact that cerebral blood is impaired in eNOS knockout mice [57,100,101]. Daily injection of atorvastatin to mice for 14 days reduced stroke volume by up to 38% in cerebral arteries by upregulation of type III NOS in aortas and in thrombocytes, and inducing NO production in both the endothelium and also, blood platelets. Thus, platelet aggregation in a thrombus was evidenced by reduced markers of platelet activity, BF 4 and β-TG. Since no alteration in these markers was observed in atorvastatin-treated eNOS knockout mice, the changes in platelet function have been attributed to the increased eNOS expression by statins [12].
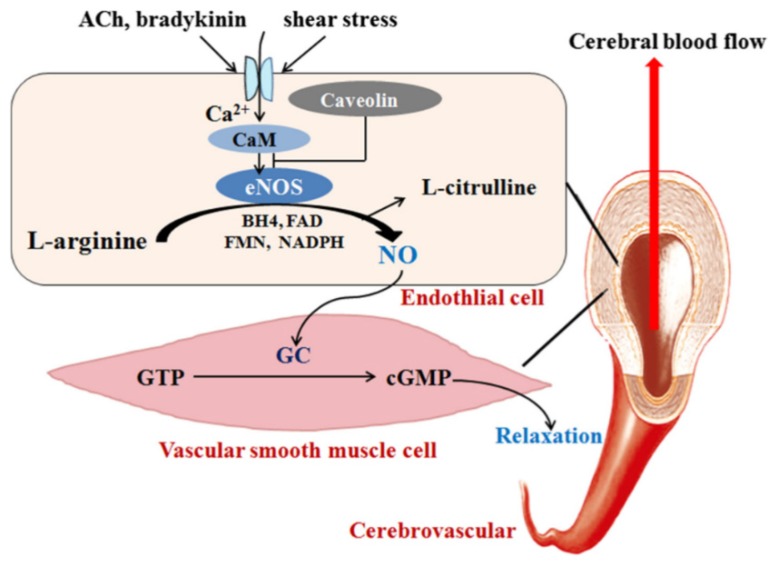
Figure 3.
eNOS and its role in the regulation of CBF. eNOS is activated by ACh, bradykinin, shear stress, etc., and then catalyzes L-arginine to generate NO which moves into vascular smooth muscle cells, reacts with GC, and promotes the conversion of GTP into cGMP, resulting in vascular smooth muscle relaxation and the CBF increase. eNOS: Endothelial oxide synthase, CBF: cerebral blood flow, Ach: Acetylcholine, NO: nitric oxide, GC: guanylate cyclase, GTP: guanosine triphosphate, cGMP: cyclic guanosine monophosphate. Reproduced with permission from [101].
3.8. Cancer Treatment
Statins have demonstrated anti-proliferative and pro-apoptotic effects in cancers. For example, a 40% risk reduction in liver cancer has been attributed to statin use by a meta-analysis [102]. Anti-cancer properties of statins are mediated either by induction of tumor cell cytotoxicity (by enhancing cytotoxic concentrations of NO) or impairing tumor angiogenesis via mechanisms independent of NO [103]. Statins increase NO concentrations through activation of inducible NOS (iNOS) which, in turn, initiates antitumor activity in macrophages and induces down-regulation of the expression of the anti-apoptotic proteins such as survivin. Therefore, transfection of tumor cells with the iNOS gene exerts antitumor effects [104,105]. Kotamraju et al. showed that simvastatin and fluvastatin induce apoptosis in breast cancer cells through production of NO mediated by iNOS so that exposure of MCF-7 breast cancer cells to sepiapterin, an eNOS activator, increases NO synthesis and improves the pro-apoptotic effects of simvastatin and fluvastatin [106]. Statins also have anti-angiogenic properties in malignant tumors through mechanisms attributed to HIF-1α inhibition via AMPK activation rather than NO increase by statins [30].
The concentration of statin is vital in determining whether anti-angiogenic or proangiogenic effects are observed. Low concentrations of cerivastatin and atorvastatin (0.005 to 0.01 µmol/L) improve angiogenesis via eNOS-activation, while high levels (0.05 to 1 µmol/L) decrease angiogenesis—which is useful for prevention of tumor angiogenesis in the treatment of cancer. The angiostatic properties of statins result from induction of apoptosis at high doses and reduction of the release of vascular endothelial growth factor (VEGF) from endothelial cells. Moreover, statin-induced reduction in GGP, a necessary molecule for Rho membrane localization and VEGF receptor activation, contributes to the anti-angiogenic properties of statins [58].
4. NO-Releasing Statins to Augment the NO-Mediated Therapeutic Effects of Statin
Introducing a NO-releasing moiety into the statin structure is an interesting idea to add to the NO-mediated therapeutic potentials of statins. For example, adding a NO-donating moiety to atorvastatin improved the ability of this statin to reduced atherosclerosis, inflammation and ROS generation [107]. The amount of released NO affects the effectiveness of these modified statins. Increased nitrosyl hemoglobin formation in the blood can be used to monitor release of NO from the statin structure. Ongini et al. incorporated a nitric ester moiety to the pravastatin and fluvastatin structure and formed more lipophilic statin derivatives that could easily penetrate the cell. Improved cell penetration led to better antiproliferative and anti-inflammatory effects; the former was confirmed by inhibited [3H] thymidine incorporation, and the later was evidenced by inhibited accumulation of nitrite, an oxidation product of NO [108]. Statins induce hepatic and muscular toxicity, as well as severe weight loss in rats suffering from hepatic cirrhosis while NCX 6560, a NO-releasing derivative of atorvastatin, reduced portal hypertension and intrahepatic vascular resistance more effectively than statins without this property of additional NO release [109].
5. Conclusions
Many pleiotropic effects of statins, including therapeutic effects in conditions including HF, hypertension, atherosclerosis, sepsis, cirrhosis, cancer, and neurologic disorders are mediated through the impact of statins on NO signaling pathways. Statins increase eNOS expression and activity and reduce NO scavenging by free radicals. Therefore, statins ameliorate NO release and bioavailability. NO is a vasodilator, promotes angiogenesis and neuroprotection, and protects the endothelium from platelet adhesion with the potential to either reduce cell apoptosis at low doses or to increase it in tumor cells at high doses. All these beneficial effects of statins, which are mediated by NO signaling, could be enhanced by modifying the chemical structure of the statin in such a way that statins release additional NO to that produced from activated eNOS. Further evidence from the clinical setting is warranted to better elucidate the interactions of statins with the NO signaling pathways, and the role of such interactions in the cardiovascular, as well as pleiotropic effects, of statins.
Abbreviations
| HMG | Hydroxymethylglutaryl |
| NO | Nitric Oxide |
| eNOS | Endothelial NO Synthase |
| ROS | Reactive Oxygen Species |
| ROCK | Rho-Associated Protein Kinase |
| PI3k | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| AMPK | AMP-Activated Protein Kinase |
| GGPP | Geranylgeranyl Pyrophosphate |
| EPC | Endothelial Progenitor Cells |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| Nox | NADPH Oxidase |
| HO-1 | Heme Oxygenase 1 |
| BH4 | Tetrahydrobiopterin |
| GTP | Guanosine-5-triphosphate |
| GTPCH | GTP Cyclohydrolase |
| CRP | C Reactive Protein |
| Cav-1 | Caveolin-1 |
| SDF-1α | Stromal Cell-Derived Factor 1 α |
| CXCR-4 | CXC Chemokine Receptor-4 |
| LV | Left Ventricle |
| CHF | Chronic Heart Failure |
| MI | Myocardial Infarction |
| cGMP | Cyclic 3,5-guanosine Monophosphate |
| ApoE | Apolipoprotein E |
| MSCs | Mesenchymal Stem Cells |
| mTOR | Mammalian Target of Rapamycin |
| BDNF | Brain-Derived Neurotrophic Factor |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor |
| iNOS | Inducible NOS |
| VEGF | Vascular Endothelial Growth Factor |
Author Contributions
All of the authors contributed with the writing, review, and editing of the study.
Funding
We are thankful for the financial support from the National Institute for Medical Research Development, Tehran, Iran (Grant no: 963401).
Conflicts of Interest
Dr. Penson owns four shares in Astra Zeneca PLC and has received travel/speaker’s fees from Amgen Inc. Dr. Banach has served on the speakers’ bureau and as an advisory board member for Amgen, Sanofi-Aventis, and Lilly. Other authors have no competing interests to disclose.
References
- 1.Banach M., Aronow W.S., Serban C., Sahabkar A., Rysz J., Voroneanu L., Covic A. Lipids, blood pressure and kidney update 2014. Pharmacol. Res. 2015;95–96:111–125. doi: 10.1016/j.phrs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Sahebkar A., Watts G.F. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther. 2013;27:559–567. doi: 10.1007/s10557-013-6479-4. [DOI] [PubMed] [Google Scholar]
- 3.Cai J., Yu X., Zhang B., Zhang H., Fang Y., Liu S., Liu T., Ding X. Atorvastatin improves survival of implanted stem cells in a rat model of renal ischemia-reperfusion injury. Am. J. Nephrol. 2014;39:466–475. doi: 10.1159/000362623. [DOI] [PubMed] [Google Scholar]
- 4.Aktas O., Albrecht P., Hartung H.P. Optic neuritis as a phase 2 paradigm for neuroprotection therapies of multiple sclerosis: Update on current trials and perspectives. Curr. Opin. Neurol. 2016;29:199–204. doi: 10.1097/WCO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 5.Chruściel P., Sahebkar A., Rembek-Wieliczko M., Serban M.C., Ursoniu S., Mikhailidis D.P., Jones S.R., Mosteoru S., Blaha M.J., Martin S.S., et al. Impact of statin therapy on plasma adiponectin concentrations: A systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. doi: 10.1016/j.atherosclerosis.2016.07.897. [DOI] [PubMed] [Google Scholar]
- 6.Parizadeh S.M.R., Azarpazhooh M.R., Moohebati M., Nematy M., Ghayour-Mobarhan M., Tavallaie S., Rahsepar A.A., Amini M., Sahebkar A., Mohammadi M., et al. Simvastatin therapy reduces prooxidant-antioxidant balance: Results of a placebo-controlled cross-over trial. Lipids. 2011;46:333–340. doi: 10.1007/s11745-010-3517-x. [DOI] [PubMed] [Google Scholar]
- 7.Sahebkar A., Kotani K., Serban C., Ursoniu S., Mikhailidis D.P., Jones S.R., Ray K.K., Blaha M.J., Rysz J., Toth P.P., et al. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015;241:433–442. doi: 10.1016/j.atherosclerosis.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Sahebkar A., Serban C., Mikhailidis D.P., Undas A., Lip G.Y.H., Muntner P., Bittner V., Ray K.K., Watts G.F., Hovingh G.K., et al. Association between statin use and plasma d-dimer levels: A systematic review and meta-analysis of randomised controlled trials. Thromb. Haemost. 2015;114:546–557. doi: 10.1160/TH14-11-0937. [DOI] [PubMed] [Google Scholar]
- 9.Kabaklic A., Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: A pilot study. Arch. Med. Sci. 2017;13:827–836. doi: 10.5114/aoms.2017.68238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta J.L., Li D.Y., Chen H.J., Joseph J., Romeo F. Inhibition of LOX-1 by statins may relate to upregulation of eNOS. Biochem. Biophys. Res. Commun. 2001;289:857–861. doi: 10.1006/bbrc.2001.6070. [DOI] [PubMed] [Google Scholar]
- 11.Kosmidou I., Moore J.P., Weber M., Searles C.D. Statin treatment and 3’ polyadenylation of eNOS mRNA. Arterioscler. Thromb. Vasc. Biol. 2007;27:2642–2649. doi: 10.1161/ATVBAHA.107.154492. [DOI] [PubMed] [Google Scholar]
- 12.Laufs U., Gertz K., Huang P., Nickenig G., Bohm M., Dirnagl U., Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.STR.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 13.Rikitake Y., Liao J.K. Rho GTPases, statins, and nitric oxide. Circ. Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laufs U., Endres M., Custodis F., Gertz K., Nickenig G., Liao J.K., Böhm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of Rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.CIR.102.25.3104. [DOI] [PubMed] [Google Scholar]
- 15.Geng J., Xu H., Yu X., Xu G., Cao H., Lin G., Sui D. Rosuvastatin protects against oxidized lowdensity lipoproteininduced endothelial cell injury of atherosclerosis in vitro. Mol. Med. Rep. 2019;19:432–440. doi: 10.3892/mmr.2018.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meda C., Plank C., Mykhaylyk O., Schmidt K., Mayer B. Effects of statins on nitric oxide/cGMP signaling in human umbilical vein endothelial cells. Pharmacol. Rep. 2010;62:100–112. doi: 10.1016/S1734-1140(10)70247-4. [DOI] [PubMed] [Google Scholar]
- 17.Rossoni L.V., Wareing M., Wenceslau C.F., Al-Abri M., Cobb C., Austin C. Acute simvastatin increases endothelial nitric oxide synthase phosphorylation via AMP-activated protein kinase and reduces contractility of isolated rat mesenteric resistance arteries. Clin. Sci. 2011;121:449–458. doi: 10.1042/CS20110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee T.-S., Chang C.-C., Zhu Y., Shyy J. Simvastatin induces heme oxygenase-1 a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 19.Piechota-Polanczyk A., Kopacz A., Kloska D., Zagrapan B., Neumayer C., Grochot-Przeczek A., Huk I., Brostjan C., Dulak J., Jozkowicz A. Simvastatin treatment upregulates HO-1 in patients with abdominal aortic aneurysm but independently of Nrf2. Oxidative Med. Cell. Longev. 2018;2018:2028936. doi: 10.1155/2018/2028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laufs U., Liao J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 21.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998;10:262–267. doi: 10.1016/S0955-0674(98)80149-X. [DOI] [PubMed] [Google Scholar]
- 22.Llevadot J., Murasawa S., Kureishi Y., Uchida S., Masuda H., Kawamoto A., Walsh K., Isner J.M., Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow–derived endothelial progenitor cells. J. Clin. Investig. 2001;108:399–405. doi: 10.1172/JCI200113131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kureishi Y., Luo Z., Shiojima I., Bialik A., Fulton D., Lefer D.J., Sessa W.C., Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Xu Z., Kitajima I., Wang Z. Effects of different statins on endothelial nitric oxide synthase and AKT phosphorylation in endothelial cells. Int. J. Cardiol. 2008;127:33–39. doi: 10.1016/j.ijcard.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Harris M.B., Blackstone M.A., Sood S.G., Li C., Goolsby J.M., Venema V.J., Kemp B.E., Venema R.C. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H560–H566. doi: 10.1152/ajpheart.00214.2004. [DOI] [PubMed] [Google Scholar]
- 26.Wolfrum S., Dendorfer A., Rikitake Y., Stalker T.J., Gong Y., Scalia R., Dominiak P., Liao J.K. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler. Thromb. Vasc. Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouet A., Sonveaux P., Dessy C., Moniotte S., Balligand J.-L., Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide; mediated effects of statins. Circ. Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 28.Sun W., Lee T.S., Zhu M., Gu C., Wang Y., Zhu Y., Shyy J.Y. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 29.Kamel W.A.-E., Sugihara E., Yamaguchi S.I., Nobusue H., Maki K., Muto A., Saya H., Shimizu T. Statins induce apoptosis in osteosarcoma cells by activation of Ampk and p38-MAPK via suppression of mevalonate pathway. Cancer Res. 2016;76:4182. doi: 10.1158/1538-7445.am2016-4182. [DOI] [Google Scholar]
- 30.Wang J.-C., Li X.-X., Sun X., Li G.-Y., Sun J.-L., Ye Y.-P., Cong L.-L., Li W.-M., Lu S.-Y., Feng J., et al. Activation of AMPK by simvastatin inhibited breast tumor angiogenesis via impeding HIF-1α-induced pro-angiogenic factor. Cancer Sci. 2018;109:1627–1637. doi: 10.1111/cas.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Q., Yang Y., Song L., Qian H., Xu Z. Atorvastatin prevents mesenchymal stem cells from hypoxia and serum-free injury through activating amp-activated protein kinase. Int. J. Cardiol. 2011;153:311–316. doi: 10.1016/j.ijcard.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Song L., Yang Y.J., Dong Q.T., Qian H.Y., Xu H., Meng X.M., Tang Y. Atorvastatin protects swine bone marrow mesenchymal stem cells from apoptosis through AMPK but not PI3K/Akt pathway. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:1033–1038. [PubMed] [Google Scholar]
- 33.Yu B., Liu D., Zhang H., Xie D., Nie W., Shi K., Yang P. Anti-hypertrophy effect of atorvastatin on myocardium depends on AMPK activation-induced miR-143-3p suppression via Foxo1. Biomed Pharmacother. 2018;106:1390–1395. doi: 10.1016/j.biopha.2018.07.064. [DOI] [PubMed] [Google Scholar]
- 34.Cerda A., Fajardo C.M., Basso R.G., Hirata M.H., Hirata R.D.C. Role of microRNAs 221/222 on statin induced nitric oxide release in human endothelial cells. Arq. Bras. Cardiol. 2015;104:195–201. doi: 10.5935/abc.20140192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J., Chen H., Ren J., Song J., Zhang F., Zhang J., Lee C., Li S., Geng Q., Cao C., et al. Effects of statin on circulating microRNAome and predicted function regulatory network in patients with unstable angina. BMC Med. Genom. 2015;8:12. doi: 10.1186/s12920-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davignon J., Jacob R.F., Mason R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004;15:251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 37.Moon G.J., Kim S.J., Cho Y.H., Ryoo S., Bang O.Y. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J. Clin. Neurol. 2014;10:140–147. doi: 10.3988/jcn.2014.10.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong H., Zhang X., Meng X., Lu L., Mai D., Qu S. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in parkinson disease models. Front. Mol. Neurosci. 2018;11:165. doi: 10.3389/fnmol.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassmann S., Laufs U., Bäumer A.T., Müller K., Konkol C., Sauer H., Böhm M., Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: Involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 40.Takeno A., Kanazawa I., Tanaka K., Notsu M., Yokomoto-Umakoshi M., Sugimoto T. Simvastatin rescues homocysteine-induced apoptosis of osteocytic MLO-Y4 cells by decreasing the expressions of NADPH oxidase 1 and 2. Endocr. J. 2016;63:389–395. doi: 10.1507/endocrj.EJ15-0480. [DOI] [PubMed] [Google Scholar]
- 41.Förstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleda S., De Haro J., Florez A., Varela C., Esparza L., Acin F. Long-term pleiotropic effect of statins upon nitric oxide and C-reactive protein levels in patients with peripheral arterial disease. Heart Asia. 2011;3:130–134. doi: 10.1136/heartasia-2011-010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez Aguilar E., De Haro Miralles J., Flórez González A., Varela Casariego C., Bleda Moreno S., Acín García F. In vivo confirmation of the Role of statins in reducing nitric oxide and C-reactive protein levels in peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 2009;37:443–447. doi: 10.1016/j.ejvs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Plenz G.A., Hofnagel O., Robenek H. Differential modulation of caveolin-1 expression in cells of the vasculature by statins. Circulation. 2004;109:e7–e8. doi: 10.1161/01.CIR.0000111128.83347.7A. [DOI] [PubMed] [Google Scholar]
- 45.Patel H.H., Murray F., Insel P.A. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stillwell W. Long-range membrane properties. In: Stillwell W., editor. An Introduction to Biological Membranes. Elsevier; Amsterdam, The Netherlands: 2016. pp. 221–245. [Google Scholar]
- 47.Gratton J.-P., Bernatchez P., Sessa W.C. Caveolae and caveolins in the cardiovascular system. Circ. Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 48.Feron O., Dessy C., Moniotte S., Desager J.P., Balligand J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelat M., Dessy C., Massion P., Desager J.P., Feron O., Balligand J.L. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/- mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 50.Mottaghi S., Larijani B., Sharifi A.M. Atorvastatin: An efficient step forward in mesenchymal stem cell therapy of diabetic retinopathy. Cytotherapy. 2013;15:263–266. doi: 10.1016/j.jcyt.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Cai A., Qiu R., Li W., Zheng D., Dong Y., Yu D., Huang Y., Rao S., Zhou Y., Mai W. Atorvastatin treatment of rats with ischemia-reperfusion injury improves adipose-derived mesenchymal stem cell migration and survival via the SDF-1α/CXCR-4 axis. PLoS ONE. 2013;8:e79100. doi: 10.1371/journal.pone.0079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell J.A., Ali F., Bailey L., Moreno L., Harrington L.S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 53.Kuhlencordt P.J., Gyurko R., Han F., Scherrer-Crosbie M., Aretz T.H., Hajjar R., Picard M.H., Huang P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 54.Strazzullo P., Kerry S.M., Barbato A., Versiero M., D’Elia L., Cappuccio F.P. Do statins reduce blood pressure: A meta-analysis of randomized, controlled trials. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 55.Golomb B.A., Dimsdale J.E., White H.L., Ritchie J.B., Criqui M.H. Reduction in blood pressure with statins: Results from the UCSD Statin Study, a randomized trial. Arch. Intern. Med. 2008;168:721–727. doi: 10.1001/archinte.168.7.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cebova M., Rehakova R., Kosutova M., Pechanova O. Simvastatin does not affect nitric oxide generation increased by sesame oil in obese Zucker rats. Oxid. Med. Cell. Longev. 2018;2018:5413423. doi: 10.1155/2018/5413423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garry P.S., Ezra M., Rowland M.J., Westbrook J., Pattinson K.T. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Weis M., Heeschen C., Glassford A.J., Cooke J.P. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 59.Bauersachs J., Galuppo P., Fraccarollo D., Christ M., Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–985. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 60.Hayashidani S., Tsutsui H., Shiomi T., Suematsu N., Kinugawa S., Ide T., Wen J., Takeshita A. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–873. doi: 10.1161/hc0702.104164. [DOI] [PubMed] [Google Scholar]
- 61.Hua P., Liu J., Tao J., Zou R., Lin X., Zhang D., Yang S. Efficacy and mechanism of preoperative simvastatin therapy on myocardial protection after extracorporeal circulation. BioMed Res. Int. 2017;2017:6082430. doi: 10.1155/2017/6082430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winzer E.B., Gaida P., Höllriegel R., Fischer T., Linke A., Schuler G., Adams V., Erbs S. Impact of Rosuvastatin Treatment on HDL-Induced PKC-βII and eNOS Phosphorylation in Endothelial Cells and Its Relation to Flow-Mediated Dilatation in Patients with Chronic Heart Failure. Cardiol. Res. Pract. 2016;2016:48261021. doi: 10.1155/2016/4826102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elmadbouh I., Mansour M., Nabeh M., Faried W., Abdelsabour A., Omar A. Atorvastatin improves cardiac function and remodeling in chronic non-ischemic heart failure: A clinical and pre-clinical study. Egypt. Heart J. 2015;67:289–298. doi: 10.1016/j.ehj.2014.11.003. [DOI] [Google Scholar]
- 64.An L., An S., Jia Z., Wang H., Yang Z., Xu C., Teng X., Wang J., Liu X., Cao Q., et al. Atorvastatin improves left ventricular remodeling and cardiac function in rats with congestive heart failure by inhibiting RhoA/Rho kinase-mediated endothelial nitric oxide synthase. Exp. Ther. Med. 2019;17:960–966. doi: 10.3892/etm.2018.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Haehling S., Anker S.D., Bassenge E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail. Rev. 2003;8:99–106. doi: 10.1023/A:1022103222857. [DOI] [PubMed] [Google Scholar]
- 66.Endres M., Laufs U., Huang Z., Nakamura T., Huang P., Moskowitz M.A., Liao J.K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szyguła-Jurkiewicz B., Szczurek W., Król B., Zembala M. The role of statins in chronic heart failure. Kardiochirurgia Torakochirurgia Polska. 2014;11:301–305. doi: 10.5114/kitp.2014.45681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westman P.C., Lipinski M.J. The use of statins in patients with heart failure: More questions than answers. J. Thorac. Dis. 2015;7:1687–1690. doi: 10.3978/j.issn.2072-1439.2015.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bielecka-Dabrowa A., Fabis J., Mikhailidis D.P., von Haehling S., Sahebkar A., Rysz J., Banach M. Prosarcopenic Effects of Statins May Limit Their Effectiveness in Patients with Heart Failure. Trends Pharmacol. Sci. 2018;39:331–353. doi: 10.1016/j.tips.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Lorkowska B., Chlopicki S. Statins as coronary vasodilators in isolated bovine coronary arteries--involvement of PGI2 and NO. ProstaglandinsLeukot. Essent. Fat. Acids. 2005;72:133–138. doi: 10.1016/j.plefa.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Koh K.K. Effects of statins on vascular wall: Vasomotor function, inflammation, and plaque stability. Cardiovasc. Res. 2000;47:648–657. doi: 10.1016/S0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 72.Vladimirova-Kitova L.G., Kitov S.I. Resistance of statin therapy, and methods for its influence. In: Kumar S.A., editor. Hypercholesterolemia. IntechOpen; London, UK: 2015. [Google Scholar]
- 73.Touyz R.M., Alves-Lopes R., Rios F.J., Camargo L.L., Anagnostopoulou A., Arner A., Montezano A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018;114:529–539. doi: 10.1093/cvr/cvy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marchio P., Guerra-Ojeda S., Vila J.M., Aldasoro M., Victor V.M., Mauricio M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019;2019:8563845. doi: 10.1155/2019/8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaric B., Obradovic M., Trpkovic A., Banach M., Mikhailidis D.P., Isenovic E. Endothelial dysfunction in dyslipidaemia: Molecular mechanisms and clinical implications. Curr. Med. Chem. 2019 doi: 10.2174/0929867326666190903112146. [DOI] [PubMed] [Google Scholar]
- 76.Ungvari Z., Csiszar A., Huang A., Kaminski P.M., Wolin M.S., Koller A. High pressure induces superoxide production in isolated arteries via protein kinase c–dependent activation of NAD (P) H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 77.Ceolotto G., Gallo A., Papparella I., Franco L., Murphy E., Iori E., Pagnin E., Fadini G.P., Albiero M., Semplicini A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD (P) H oxidase via AMPK-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 78.Pereira E.C., Bertolami M.C., Faludi A.A., Salem M., Bersch D., Abdalla D.S.P. Effects of simvastatin and l -arginine on vasodilation, nitric oxide metabolites and endogenous NOS inhibitors in hypercholesterolemic subjects. Free Radic. Res. 2003;37:529–536. doi: 10.1080/1071576031000083170. [DOI] [PubMed] [Google Scholar]
- 79.Lee S.-E., Chang H.-J., Sung J.M., Park H.-B., Heo R., Rizvi A., Lin F.Y., Kumar A., Hadamitzky M., Kim Y.J., et al. Effects of statins on coronary atherosclerotic plaques. JACC Cardiovasc. Imaging. 2018;11:1475–1484. doi: 10.1016/j.jcmg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Bittencourt M.S., Cerci R.J. Statin effects on atherosclerotic plaques: Regression or healing? BMC Med. 2015;13:260. doi: 10.1186/s12916-015-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heeba G., Moselhy M.E., Hassan M., Khalifa M., Gryglewski R., Malinski T. Anti-atherogenic effect of statins: Role of nitric oxide, peroxynitrite and haem oxygenase-1. Br. J. Pharm. 2009;156:1256–1266. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rasmusen C., Cynober L., Couderc R. Arginine and statins: Relationship between the nitric oxide pathway and the atherosclerosis development. Ann. Biol. Clin. 2005;63:443–455. [PubMed] [Google Scholar]
- 83.McGown C.C., Brookes Z.L.S. Beneficial effects of statins on the microcirculation during sepsis: The role of nitric oxide. Br. J. Anaesth. 2007;98:163–175. doi: 10.1093/bja/ael358. [DOI] [PubMed] [Google Scholar]
- 84.Zafra C., Abraldes J.G., Turnes J., Berzigotti A., Fernández M., García-Pagán J.C., Rodés J., Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Abraldes J.G., Rodríguez-Vilarrupla A., Graupera M., Zafra C., García-Calderó H., García-Pagán J.C., Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J. Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 86.Trebicka J., Hennenberg M., Laleman W., Shelest N., Biecker E., Schepke M., Nevens F., Sauerbruch T., Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan D.E. The Use of Statins in Patients with Cirrhosis. Gastroenterol. Hepatol. 2018;14:485–487. [PMC free article] [PubMed] [Google Scholar]
- 88.Chu H., Wang Y. Therapeutic angiogenesis: Controlled delivery of angiogenic factors. Ther. Deliv. 2012;3:693–714. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J., Cheng M., Liao Y.-H., Hu Y., Wu M., Wang Q., Qin B., Wang H., Zhu Y., Gao X.-M., et al. Rosuvastatin Enhances Angiogenesis via eNOS-Dependent Mobilization of Endothelial Progenitor Cells. PLoS ONE. 2013;8:e63126. doi: 10.1371/journal.pone.0063126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urbich C., Dernbach E., Zeiher A.M., Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ. Res. 2002;90:737–744. doi: 10.1161/01.RES.0000014081.30867.F8. [DOI] [PubMed] [Google Scholar]
- 91.Llevadot J., Asahara T. Effects of statins on angiogenesis and vasculogenesis. Rev. Esp. Cardiol. 2002;55:838–844. doi: 10.1016/S0300-8932(02)76713-4. [DOI] [PubMed] [Google Scholar]
- 92.Yu W.-L., Sun T.-W., Qi C., Zhao H.-K., Ding Z.-Y., Zhang Z.-W., Sun B.-B., Shen J., Chen F., Zhu Y.-J., et al. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration. Sci. Rep. 2017;7:44129. doi: 10.1038/srep44129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H., Li W., Liu C., Tan J., Wang H., Hai B., Cai H., Leng H.-J., Liu Z.-J., Song C.-L. Incorporating simvastatin/poloxamer 407 hydrogel into 3D-printed porous Ti6Al4V scaffolds for the promotion of angiogenesis, osseointegration and bone ingrowth. Biofabrication. 2016;8:045012. doi: 10.1088/1758-5090/8/4/045012. [DOI] [PubMed] [Google Scholar]
- 94.Khaidakov M., Wang W., Khan J.A., Kang B.Y., Hermonat P.L., Mehta J.L. Statins and angiogenesis: Is it about connections? Biochem. Biophys. Res. Commun. 2009;387:543–547. doi: 10.1016/j.bbrc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 95.Zemankova L., Varejckova M., Dolezalova E., Fikrova P., Jezkova K., Rathouska J., Cerveny L., Botella L.M., Bernabeu C., Nemeckova I., et al. Atorvastatin-induced endothelial nitric oxide synthase expression in endothelial cells is mediated by endoglin. J. Physiol. Pharm. 2015;66:403–413. [PubMed] [Google Scholar]
- 96.Zhang J., Wang H., Ye P. Effect of atorvastatin on eNOS synthesis in organs of aging rats with myocardial ischemia-reperfusion. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1708–1712. [PubMed] [Google Scholar]
- 97.Yang Y., Yin D., Wang F., Hou Z., Fang Z. In situ eNOS/NO up-regulation—A simple and effective therapeutic strategy for diabetic skin ulcer. Sci. Rep. 2016;6:30326. doi: 10.1038/srep30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao K., Wang G., Wang Y., Han D., Bi J., Yuan Y., Yao T., Wan Z., Li H., Mei X. Neuroprotective effect of simvastatin via inducing the autophagy on spinal cord injury in the rat model. BioMed Res. Int. 2015;2015:260161. doi: 10.1155/2015/260161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelly P., Prabhakaran S. Statins for neuroprotection after acute ischemic stroke. Stroke. 2017;48:2922–2923. doi: 10.1161/STROKEAHA.117.018725. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z.-Q., Mou R.-T., Feng D.-X., Wang Z., Chen G. The role of nitric oxide in stroke. Med. Gas Res. 2017;7:194–203. doi: 10.4103/2045-9912.215750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu J., Song W., Li L., Fan X. Endothelial nitric oxide synthase: A potential therapeutic target for cerebrovascular diseases. Mol. Brain. 2016;9:30. doi: 10.1186/s13041-016-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi M., Zheng H., Nie B., Gong W., Cui X. Statin use and risk of liver cancer: An update meta-analysis. BMJ Open. 2014;4:e005399. doi: 10.1136/bmjopen-2014-005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fukumura D., Kashiwagi S., Jain R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 104.Le X., Wei D., Huang S., Lancaster J.R., Xie K. Nitric oxide synthase II suppresses the growth and metastasis of human cancer regardless of its up-regulation of protumor factors. Proc. Natl. Acad. Sci. USA. 2005;102:8758–8763. doi: 10.1073/pnas.0409581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao J.-I., Kuo P.-C., Hsu T.-S. Down-regulation of survivin in nitric oxide-induced cell growth inhibition and apoptosis of the human lung carcinoma cells. J. Biol. Chem. 2004;279:20267–20276. doi: 10.1074/jbc.M312381200. [DOI] [PubMed] [Google Scholar]
- 106.Kotamraju S., Williams C.L., Kalyanaraman B. Statin-induced breast cancer cell death: Role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67:7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 107.Momi S., Monopoli A., Alberti P.F., Falcinelli E., Corazzi T., Conti V., Miglietta D., Ongini E., Minuz P., Gresele P. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovasc. Res. 2012;94:428–438. doi: 10.1093/cvr/cvs100. [DOI] [PubMed] [Google Scholar]
- 108.Ongini E., Impagnatiello F., Bonazzi A., Guzzetta M., Govoni M., Monopoli A., Del Soldato P., Ignarro L.J. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc. Natl. Acad. Sci. USA. 2004;101:8497–8502. doi: 10.1073/pnas.0401996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodríguez S., Raurell I., Torres-Arauz M., García-Lezana T., Genescà J., Martell M. A Nitric Oxide-Donating Statin Decreases Portal Pressure with a Better Toxicity Profile than Conventional Statins in Cirrhotic Rats. Sci. Rep. 2017;7:40461. doi: 10.1038/srep40461. [DOI] [PMC free article] [PubMed] [Google Scholar]