Figure 4.
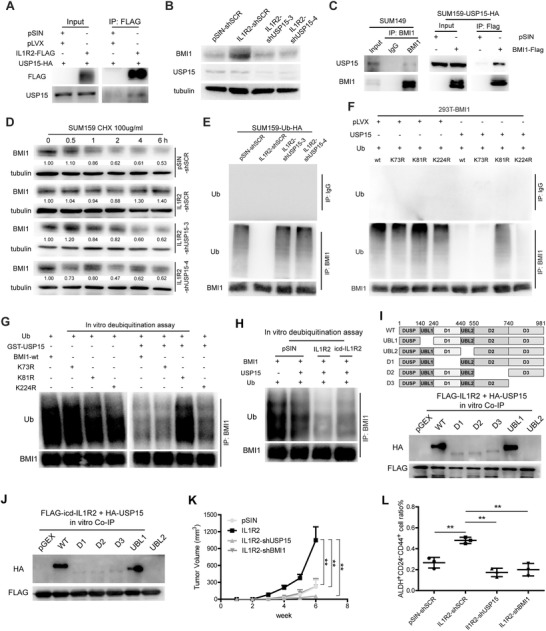
IL1R2 regulated BMI1 deubiquitination via interaction with USP15. A) IL1R2 protein interacted with USP15 in SUM159 cells in the Co‐IP assay. B) Knockdown of USP15 inhibited BMI1 protein expression in SUM159‐IL1R2 cells. C) Co‐IP analysis results showed that USP15 could interact with BMI1 protein in SUM159 cells. D) Knockdown of USP15 promoted BMI1 protein degradation in SUM159‐IL1R2 cells after CHX treatment. E) Silencing of USP15 promoted the ubiquitination of BMI1 protein in IL1R2‐overexpressing cells. F) Ubiquitin, USP15, wild‐type BMI1 and its mutant (K73R, K81R, and K224R) were co‐overexpressed in 293T cells, and Co‐IP analysis showed that USP15 deubiquitinated BMI1 wild type, BMI1‐K73R, and BMI1‐K224R mutant but not in BMI1‐K81R mutant. G) Ub and BMI1 or its mutant (BMI1‐K73R, BMI1‐K81R, or BMI1‐K224R) were cotransfected in 293T cell, and ubiquitinated BMI1 proteins were purified as the ubiquitinated substrates under denaturing conditions. GST‐fusion USP15 were purified from BL21 strain and precipitated by GST sepharos. The ubiquitination status of BMI1 was analyzed by Western blotting assay. H) In vitro deubiquitination assay results showed that addition of GST‐fusion IL1R2 or icd‐IL1R2 protein significantly enhanced the activity of USP15 on BMI1 dequbiquitination. I) IL1R2 and USP15 protein truncations were purified from BL21 strain and Co‐IP assay were carried out in vitro. J) icd‐IL1R2 and USP15 protein truncations were purified from BL21 strain and Co‐IP assay were carried out in vitro. K,L) Knockdown of USP15 or BMI1 in IL1R2‐overexpressing SUM159 cells inhibited its xenografts growth and BTICs enrichment in vivo (**, p < 0.01 vs the group indicated).
