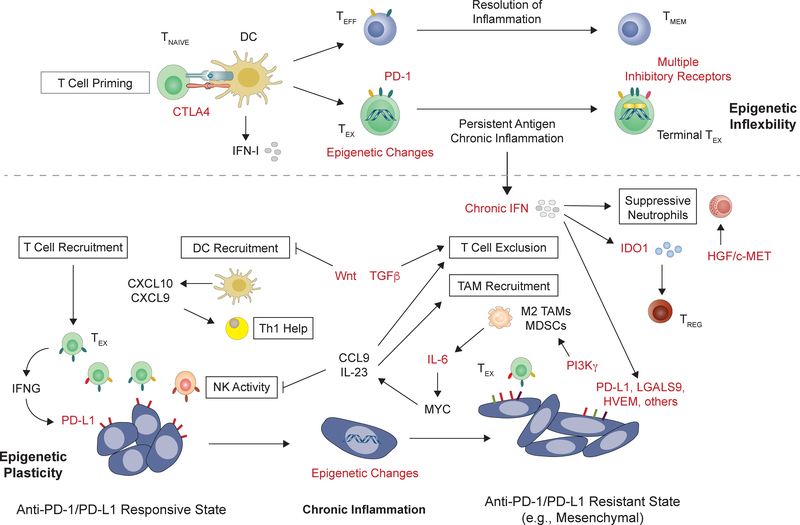
Figure 4: Targets to interfere with feedback inhibition mechanisms from immune cells and the tumor microenvironment.
Naïve T cells (TNAIVE) undergo different cell fates depending on the nature of the antigen and the course of inflammation (above dashed line). After T cell priming, persistent antigen and chronic inflammation, which typifies cancer, results in T cell exhaustion as a mechanism to limit immune-mediated pathology. In contrast to effector T cells (TEFF) that differentiate into memory T cells (TMEM), these exhausted T cells (TEX) have poor effector function that may be epigenetically “locked-in”. Targeting multiple co-expressed T cell inhibitory receptors can improve TEX function. In contrast to TEX, the tumor microenvironment (TME) generally demonstrates plasticity (below dashed line). Instructed by aberrant environmental cues and feedback signals associated with chronic inflammation and non-healing wounds, cancers can acquire various immune-TME phenotypes (boxed in black). DC and T cell recruitment favor response to PD1–1/PD-L1 blockade. However, tumors that transition into mesenchymal states by inflammatory cytokines or oncogenic signals exhibit an immune-TME that excludes T cells and supports suppressive TAMs and MDSCs. Chronic cytokine signals such as IFN can exacerbate immune suppression through various mechanisms, which can contrast with their typical stimulatory roles during productive immune responses that resolve. When multiple immune suppressive mechanisms dominate, resistance or relapse to anti-PD-1/PD-L1 typically occurs. Potential targets for combination ICB therapy is indicated in red and include the epigenetic state of tumor and immune cells, intracellular and extracellular signaling pathways, suppressive cytokines, and non-redundant T cell inhibitory receptor pathways. See Table 2 for examples of agents against these targets.