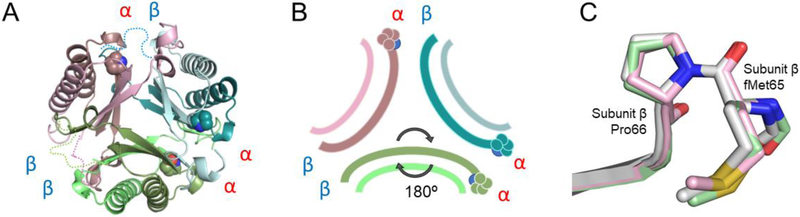
Figure 5.
A) Ribbon diagram of unfused 4-OT. Heterodimer A colored two shades of green, heterodimer B is colored two shades of teal and heterodimer C colored taupe/pink. The arrangement is an asymmetric trimer of dimers. The interfaces remain αα, αβ, and ββ, as labeled. The missing linker loop for each dimer is shown in dotted lines in corresponding colors. B) Schematic diagram of unfused 4-OT showing the flipped dimer and the corresponding interfaces. C) Overlay of three β-subunits of unfused 4-OT showing stick mode of the first three amino acids. The Leu-Pro sequence in the fused 4-OT is replaced by the initiating methionine followed by proline (Pro-66 in the fused 4-OT), in all β-subunits of the unfused 4-OT. The Met-Pro is in the trans configuration.