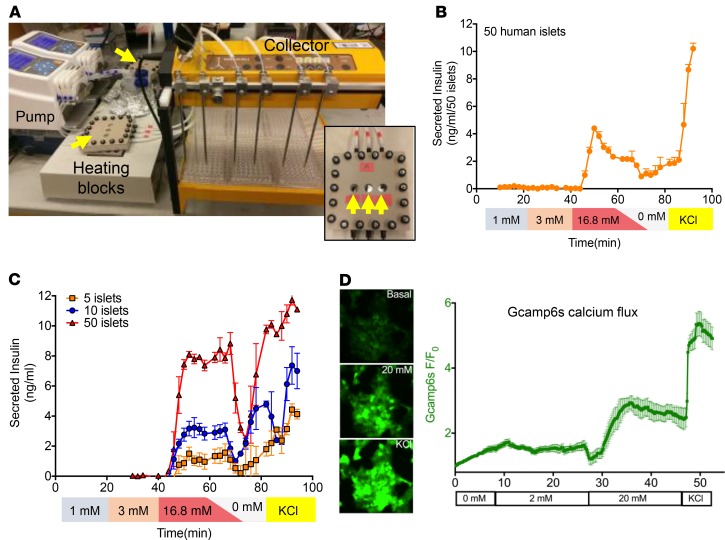
Figure 1. Custom multichannel perifusion system with real-time imaging capacity.
(A) The multichannel perifusion system, consisting of two peristaltic pumps, two 3-channel perifusion chambers (yellow arrows), heating blocks, tubing, and a 6-channel fraction collector. Inset: Enhanced view of an individual 3-channel perifusion chamber (yellow arrows indicate optical chambers). (B) Measurement of GSIS using the custom multichannel perifusion system. Fifty human islets per channel, each from the same donor, were perfused in triplicate with 1 mM, 3 mM, and 16.8 mM glucose, followed by a short period of KRBB buffer and 30 mM KCl (see Methods for details). Fractions were collected every minute, and insulin concentrations were measured by HTRF insulin assay. Experiments were repeated 10 times with islets isolated from donors shown in Table 1. Results from a single representative experiment are shown. Each data point represents the mean ± SEM of insulin measurement (n = 3 replicates per time point). (C) Sensitivity of the multichannel perifusion system. Five, 10, or 50 human islets from the same donor were loaded into each well of a perifusion chamber, followed by perifusion as described in B. Each curve represents a single experiment (n = 3 replicates per time point). Each data point represents mean ± SEM of insulin measurement. (D) Real-time imaging analysis of calcium influx in EndoC-βH1 cells in response to glucose stimulation. EndoC-βH1 cells were engineered to express a calcium-sensitive green fluorescence reporter (GCaMP6s) under the control of the rat insulin promoter. Cells were placed into the perifusion chamber, mounted on an inverted epifluorescence microscope, and subjected to perifusion using the indicated concentrations of glucose and KCl (see Methods). Experiments were performed in triplicate and results plotted as mean fluorescence intensity of the whole field versus time (sampling every 10 seconds).