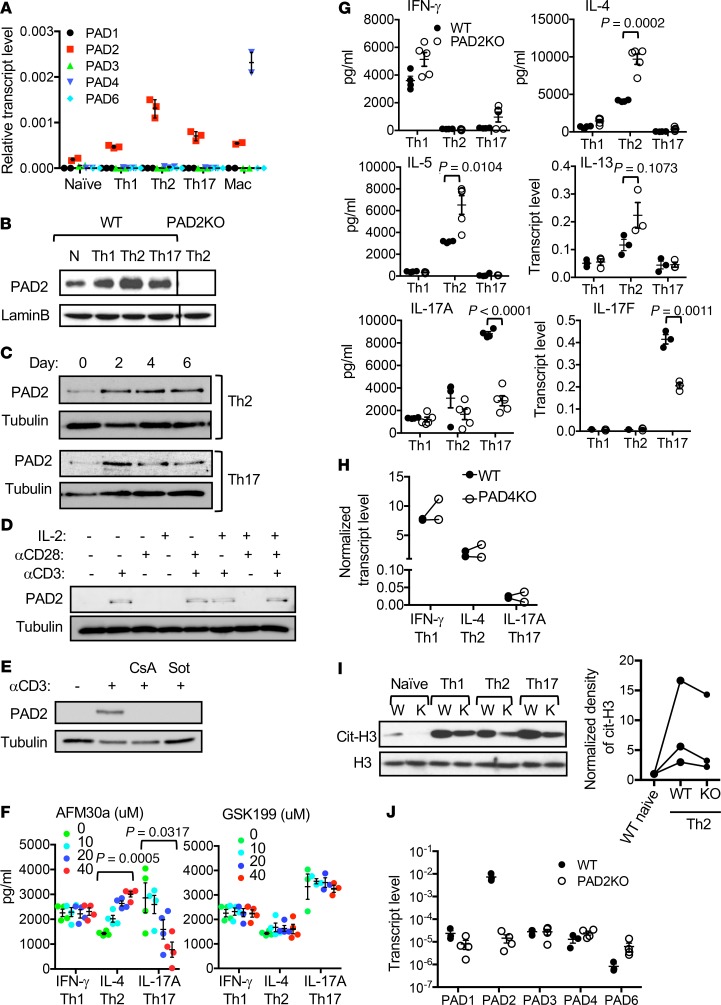
Figure 2. Recapitulating the effects of Cl-am by inhibiting PAD2 but not PAD4.
(A) The transcript levels of indicated PADs in Th cells (n = 3) and macrophages (n = 2) of C57BL/6 mice were measured with real-time PCR and normalized against the levels of actin. (B) The levels of PAD2 proteins in indicated C57BL/6 Th cells and PAD2-KO Th2 cells were examined with Western blotting using indicated antibodies. (C) At indicated time points during the differentiation of WT Th2 and Th17 cells, the level of PAD2 protein was examined with Western blotting. A parallel gel was probed with anti-tubulin for loading control. (D and E) WT Th cells were stimulated with anti-CD3, anti-CD28, and/or IL-2 in the absence (D) or presence (E) of cyclosporine A (CsA) or sotrastaurin (Sot) for 2 days. The level of PAD2 proteins was examined with Western blotting. Representative Western blots from 2 experiments are shown in B–D. (F) C57BL/6 effector Th cells were generated in the presence of AFM30a (left panel) or GSK199 (right panel) at indicated concentrations. The expression of indicated cytokines by the Th cells after restimulation with anti-CD3 is shown (n = 3–4, 1-way ANOVA). (G–J) Th cells obtained from WT DBA/1J (G–J, n = 3–4), PAD2-KO (G, I, and J, n = 3–5), or PAD4-KO (H, n = 2) mice were differentiated into Th1, Th2, and Th17 cells for 5 days. The production of indicated cytokines after restimulation with anti-CD3 was measured with ELISA or real-time PCR (G and H, 2-tailed Student’s t test for G). Data from the same experiments are connected with lines in H. Whole cell extract from WT DBA/1J or PAD2-KO Th cells were examined with Western blotting using anti-citH3 (I). A parallel gel was probed with anti-H3 for loading controls. The normalized density of cit-H3 of indicated cells is shown in the dot blot of I. The transcript levels of PADs in restimulated WT DBA/1J, and PAD2-KO Th2 cells were quantified with real-time PCR and shown in J (n = 3–4).