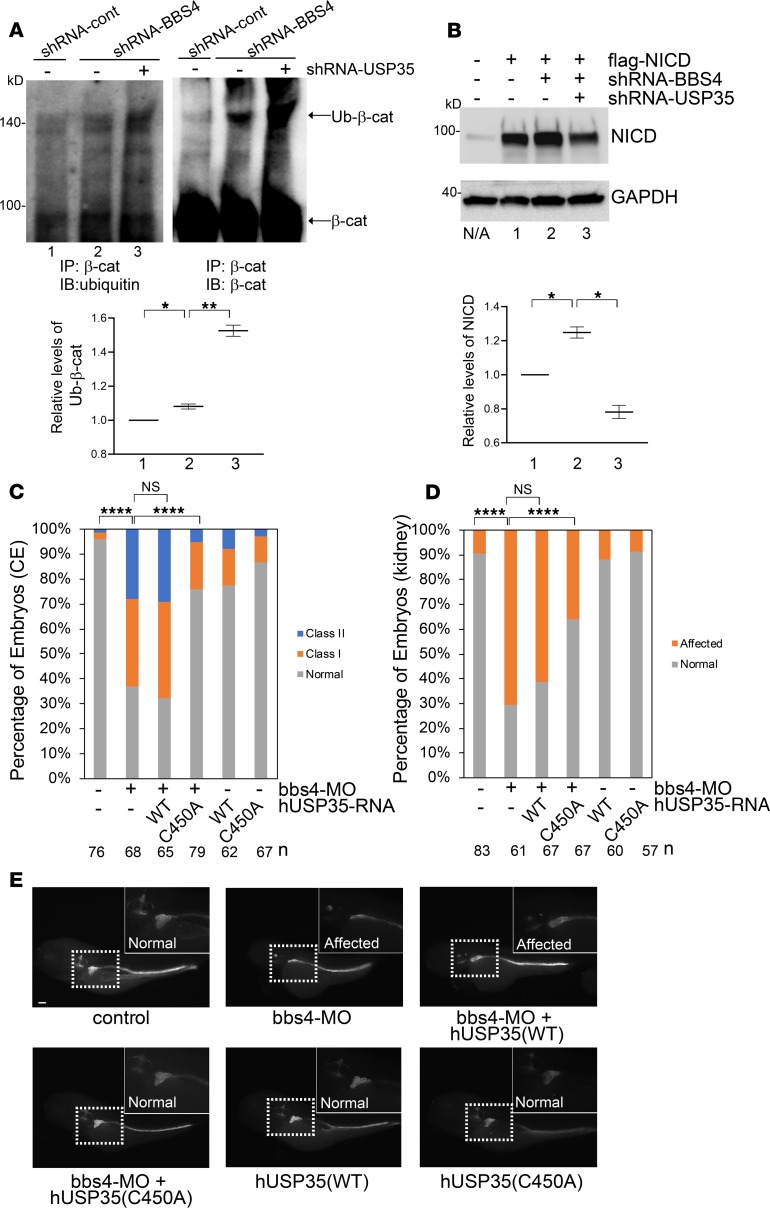
Figure 3. The molecular mechanism by which USP35 suppression ameliorate the defects caused by BBS4 depletion.
(A) Suppression of USP35 enhances the ubiquitination of β-catenin. BBS4-depleted HEK293 cells were transfected with either shRNA-control or shRNA targeting to USP35. 72 hours after transfection, immunoprecipitation of β-catenin was performed, followed by immunoblotting with β-catenin and ubiquitin. The relative quantification of β-catenin ubiquitination was performed with QuantityOne. *P < 0.05, **P < 0.01 (2-tailed Student’s t test). (B) USP35 suppression facilitated the degradation of accumulated NICD. HEK293 cells were transfected with the plasmids as indicated in the figure. Seventy-two hours after transfection, cells were harvested and subjected to immunoblotting of NICD and GAPDH (loading control). Relative levels of NICD from 2 experiments are indicated in the bottom of the panel. *P < 0.05 (2-tailed Student’s t test) (C) Coexpression of dominant negative human USP35 in bbs4 morphants ameliorates CE defects. Zebrafish embryos were injected with bbs4 morpholino and mRNA encoding either WT or dominant negative human USP35. CE defects can be ameliorated by coexpression of hUSP35 (C450A) but not hUSP35 (WT). ****P < 0.0001 (χ2 analysis). (D) Loss of bbs4 leads to the atrophy and deficit convolution in the proximal tubule. Coexpression of hUSP35 (C450A) but not hUSP35 (WT) can ameliorate the renal development defects. ****P < 0.0001 (χ2 analysis). (E) Represented images of D. Scale bar: 100 μm.