Figure 1.
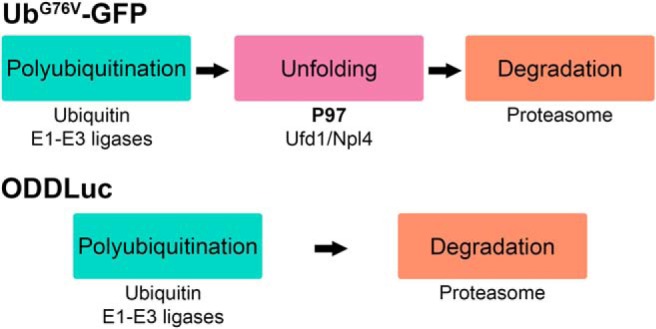
The in vivo proteasomal activity reporters UbG76V-GFP and ODDLuc are degraded through P97-dependent and P97-independent mechanisms. Top, UbG76V-GFP is a fusion protein consisting of GFP fused to a non-cleavable molecule of ubiquitin, which acts as a constitutively active degradation signal. UbG76V-GFP is degraded by proteasomes following its polyubiquitination and partial unfolding by the P97-Ufd1-Npl4 complex. Bottom, ODDLuc is a fusion protein consisting of firefly luciferase fused to an alternative degradation signal, the ODD of HIF1α. This reporter is polyubiquitinated and degraded by proteasomes without P97 processing. Conditions of inhibited, impaired, or insufficient UPS function result in the intracellular accumulation of either reporter.
