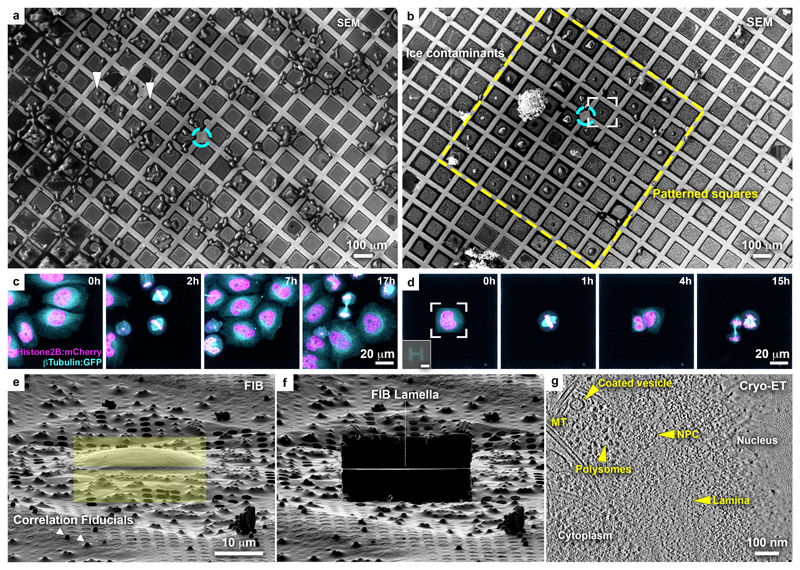
Figure 1. Micropatterning of cryo-EM grids refines preparation for cryo-FIB lamella micromachining from adherent mammalian cells.
(a) Cryo-scanning electron micrograph (SEM) of HeLa cells grown overnight on a gold-mesh grid with a holey (R2/1) SiO2 film. Cyan circle indicates grid center. Only a small fraction of the cells is optimally positioned for FIB-lamellae preparation (arrowheads). (b) HeLa cells grown overnight on a gold-mesh grid coated with a holey (R2/1) carbon film micropatterned with 20 μm diameter disks on 8 x 8 grid squares (yellow rectangle) around the grid center (cyan circle) and treated with fibronectin. (c, d) HeLa cells, expressing GFP-tagged β-tubulin (Cyan) and mCherry-tagged histone (H2B: magenta), seeded on a (c) control and (d) patterned gold-mesh grids with SiO2 (R1.2/20) holey film. Inset: H-shaped pattern induced the square cell shape. Scale: 20 μm. Cell-cycle was synchronized with a single Thymidine block (16 h), released into fresh medium (8h), followed by overnight live-cell imaging. A field of view of a single grid square is shown for both (c) and (d). Cells continue dividing ~40 h post-seeding (see Supplementary Video 1). (e) FIB shallow angle view on cell framed in (b). Yellow rectangles indicate patterns for milling. Correlation fiducials (microbeads; arrowheads) can facilitate 3D-targeted cryo-FIB preparations. (f) Lamella produced from cell in (e). (g) Tomographic slice, 6.8 nm thickness, of the nuclear periphery of the cell in (e). Lamella thickness determined from the tomographic reconstruction was 90 nm. NPC: nuclear pore complex; MT: microtubule.