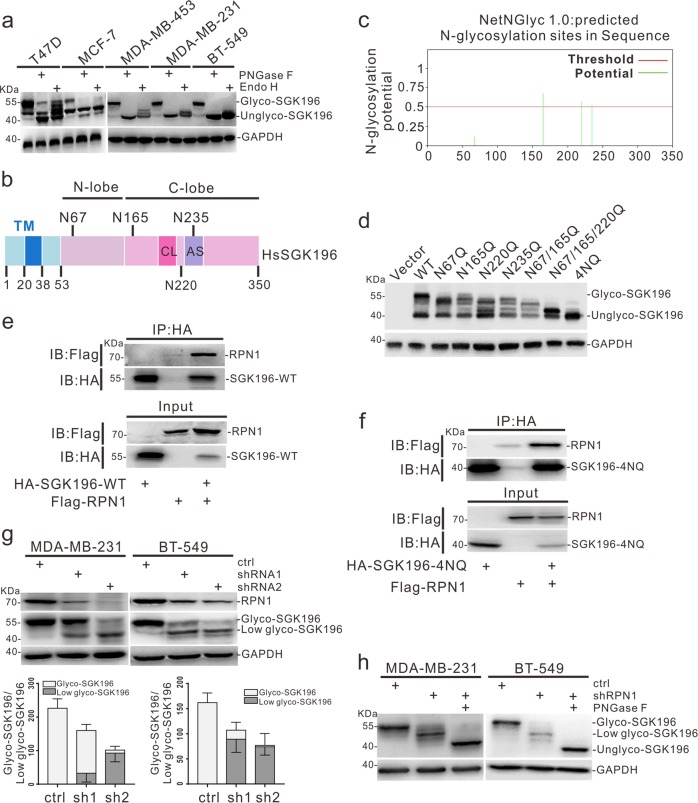
Fig. 2. SGK196 is modified via N-linked glycosylation in BC cells and partially regulated by RPN1.
a Cell lysates from different subtypes BC cell lines were treated with PNGase F or Endo H, and then were used for Western blotting analysis of SGK196 protein. b Schematic illustration of human SGK196 protein and its potential N-glycosylation sites. c Predicted N-glycosylation sites of human SGK196 by NetNGlyc1.0 Server. d Cell lysates from HEK293 cells transiently transfected with HA-tagged SGK196-WT or -mutants were collected and subjected to Western blotting analysis. 4NQ represents N67/165/220/235Q mutation. e, f Co-immunoprecipitation analysis in HEK293 cells revealing that RPN1 interacts with both SGK196-WT (e) and SGK196-4NQ (f). g Western blotting analysis of glycosylation status of SGK196 in MDA-MB-231 cells and BT-549 cells infected with control shRNA and RPN1 shRNA and the relative ratio of the upper to the lower form of SGK196 was quantified when RPN1 expression was diminished in MDA-MB-231 cells and BT-549 cells. h Western blotting analysis of glycosylation status of SGK196 in MDA-MB-231 cells and BT-549 cells infected with RPN1 shRNA and control shRNA; molecular weight shifts are compared with or without PNGase F digestion treatment.