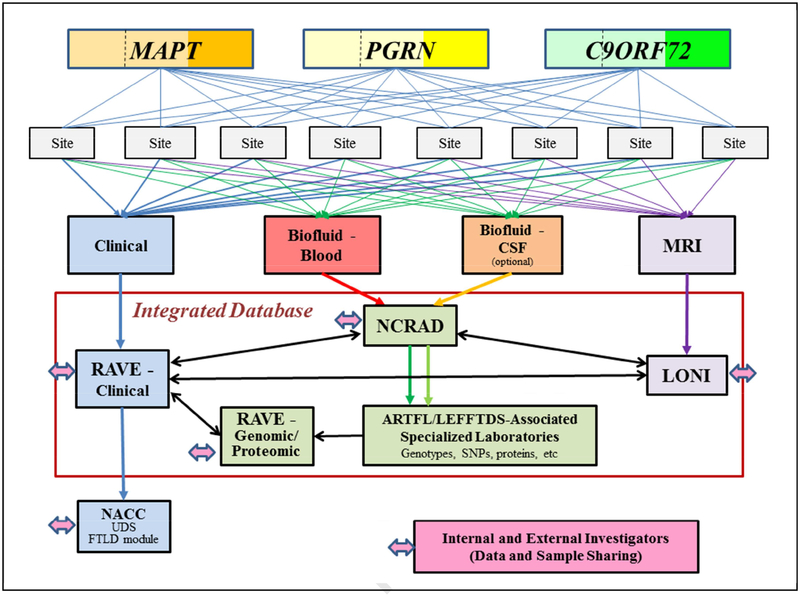
Figure 2. Protocol Schema.
Three hundred subjects (100 among kindreds with a mutation in MAPT, 100 among kindreds with a mutation in PGRN, and 100 among kindreds with the C9orf72 mutation) are enrolled and followed at one of the eight sites. Within each gene, approximately 1/3 are symptomatic (reflected by darker shades of orange, yellow or green) whereas 2/3 are asymptomatic (reflected by lighter shades of the colors). The lighter shade areas are divided by a dashed line, which reflects one half of the asymptomatic are non-mutation carrier/family controls while the other half are mutation carriers. Each subject can participate in four research arms – clinical, biofluid-blood, biofluid-CSF and MRI; the CSF arm is optional. Each subject can also participate in a 5th arm (not shown) in which clinical genetic counseling and testing can be performed. The clinical data is entered into an electronic data capture system (RAVE), and the majority of this data is uploaded to the NACC. Biofluid samples are submitted to NCRAD for processing and storage. See text for abbreviations.