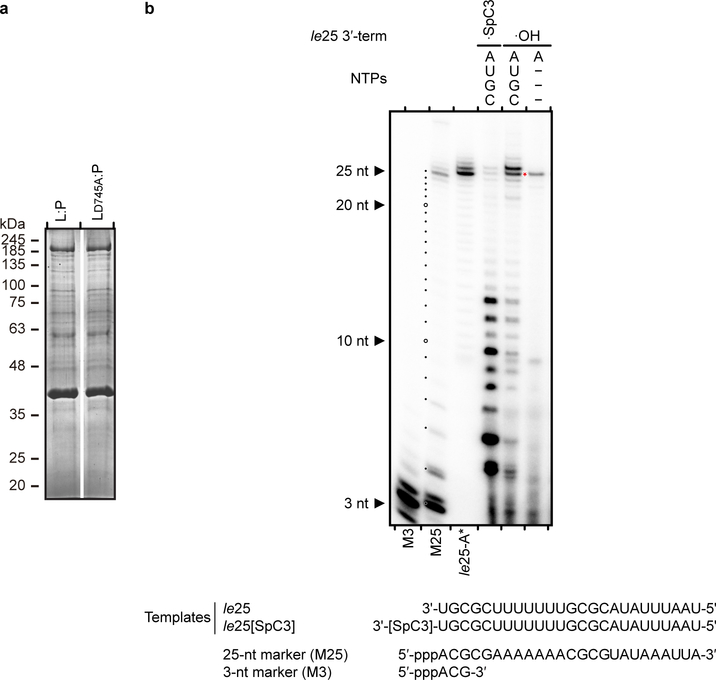
Extended Data Fig. 2 |. RdRp activity assay.
a, SDS PAGE of HMPV wild type L:P, LD745A:P purified for RdRp activity assays. Proteins were purified by metal affinity, TEV cleavage of Histidine-tag followed by reverse His-tag affinity purification and size exclusion chromatography. b, Analysis of the 3′ extension activity of HMPV polymerase using the le25 RNA template. Reactions were performed with rNTPs (0.5 mM each of rUTP, rGTP and rCTP), 20 μM rATP and 20 nM [α-32P] rATP. When a 3’-modified le25 (le25[SpC3], three-carbon spacer group linked to the 3’ extremity) was used as a template, synthesis of products greater than 25 nt was greatly reduced compared to le25. When only [α-32P]rATP and no other rNTP was supplied, only a product with size greater than 25 nt was observed. This result shows that the L:P complex was capable of modifying the 3’ terminus of the template, in addition to engaging in de novo initiation at the promoter. The radiolabeled RNA products were visualized by phosphorimaging. Data are representative of three independent experiments. For gel source data, see Supplementary Fig. 1.