Abstract
Although there is a high risk of mood disorders and cognitive impairment in congenital human cytomegalovirus (HCMV) infections, the molecular pathogenetic mechanisms of HCMV have not yet been fully determined. In this study, we show that immediate-early 2 (IE2) protein modulates affective and cognitive behaviors. We used a UL122 genetically-modified mice model that can continuously express IE2 protein. We used a series of animal behavior tests to determine the relationship between HCMV-encoded IE2 and psychiatric disorders. In open-field, elevated plus-maze test and tail suspension tests, we found that UL122 genetically-modified mice displayed more anxiety-depression behavior than did wild-type (WT) mice. The Morris water maze test and novel object recognition test showed that spatial learning and memory were lower in UL122 genetically-modified mice model than in WT mice. Level of fibroblast growth factor 2 (FGF2) protein in the hippocampus cornu ammonia areas (CA1, CA3) and dentate gyrus (DG) of the experimental group was significantly lower, consistent with immunohistochemical staining and western blot for neuron-specific nuclear protein (NeuN) and glial fibrillary acidic protein (GFAP). Levels of SYP and PSD-95 proteins were lower in the hippocampus UL122 genetically-modified mice. These data suggest the importance of HCMV-encoded IE2 for studying anxiety and depression behaviors and for the spatial learning and memory. This would help to further explain the molecular pathological mechanism of psychiatric disorders caused by HCMV infection.
Keywords: IE2, anxiety-depression, cognitive impairment, synaptic plasticity, mice
Introduction
Anxiety-depression and cognitive impairment affect millions of people worldwide. The suicide rate is much higher for people with mood disorders than for the general population [1]. Cognitive impairment, including impaired learning and memory deterioration, are implicated in neurological diseases such as Alzheimer’s [2]. The molecular mechanisms of anxiety-depression and cognitive impairment are closely related to synaptic plasticity [3].
Human cytomegalovirus (HCMV) is a double-stranded DNA virus that belongs to the family Herpesviridae and subfamily Betaherpesvirinae [4]. The primary target of HCMV is the hippocampus, a key brain region involved in memory and emotional processing. Several studies have shown that HCMV infection may lead to long-term neurodevelopmental impairment that may in turn cause neurological disorders and intellectual impairment [5]. Several studies have demonstrated that congenital HCMV infection induces cognitive impairment by inhibiting the synaptic plasticity of the mice [6,7]. A recent study suggests that mood disorders, such as depression and anxiety, may be associated with HCMV infection [8].
HCMV generates two major viral gene products, immediate early IE1 and IE2 proteins; these are expressed at the highest levels during the viral stage of replication [9]. IE2 is encoded by the gene UL122; it is the most important protein with respect to HCMV latency and replication [10]. Because of the highly species-specific nature of HCMV, the study of IE2 is limited to in vitro models of infection. The establishment of UL122 overcame this species specificity and provided an effective method to study the influence of IE2 on symptoms of depression, anxiety, and cognitive impairment. This animal model can be used to study HCMV infection and contribute to understanding the mechanism by which IE2 participates in pathogenesis, as well as help to provide a theoretical basis for the prevention and treatment of various diseases.
Despite the substantial evidence that HCMV infection triggers mood disorders and cognitive impairment by inhibiting synaptic plasticity, the crucial role IE2 played in HCMV-caused psychiatric disorders remains to be identified. Therefore, we investigated whether HCMV-encoded IE2 affected mood and cognitive-related behaviors in UL122 transgenic mice. A series of animal behavior tests were used to assess potential links between HCMV-encoded IE2 and mood disorders and cognitive impairments.
Materials and methods
Animals
Four UL122 genetically-modified mice, two female and two male that constitutively express IE2 were obtained from the Laboratory of Pathogenic Biology of Qingdao University. All animal experiments were authorized by the Animal Experiments Committee of Qingdao University. We extracted the DNA from the tails of two-week-old mice. Subsequently, the UL122 genetically-modified mice were verified using PCR technology. UL122-positive mice were categorized as the experimental group and the negatives were the controls.
DNA extraction and PCR
DNA extraction was prepared from each mouse tail using a DNeasy tissue Kit (TIANGEN). The cycling condition details of HCMV IE2 gene were as follows: Pre-denaturation at 94°C for 5 min and then 35 cycle of 94°C, 30 s; annealing at 60°C for 35 s; extension at 72°C for 1 min and further at 72°C for 10 min. The primer sequences were 5’-3’: CAGTCCGCCCTGAGCAAAGA (Forward) and 5’-3’: TATGAACAAACGACCCAACAC-CC (Reverse).
Open-field test (OF)
The mice were placed in the middle of the enclosed testing chamber divided into center and periphery. Five-minute testing behaviors in the center and periphery of the chamber were recorded using Ethovision software with a camera-driven tracker system. In the open field test, the time that was spent in the center and periphery of the arena were used to evaluate anxious behavior. After each test, the instrument was cleaned with 70% alcohol to avoid interference.
Elevated plus-maze test (EPM)
Anxiety-like behavior was evaluated using the elevated plus-maze test, consisting of two exposed arms and two closed arms. The four arms were linked using a neutral platform. Initially, every mouse was positioned in the junction area facing a closed arm and was permitted to freely explore for 5 minutes. The time spent in the open and closed arms was recorded as a proxy for anxious behavior.
Tail suspension test (TST)
TST is a depressive-like behavior model, in which immobility suggests desperate behavior under stressful circumstance. We suspended mice separately for 6 min on the rim of a rod 60 cm above the floor. When they were hung down inactively, the mice were regarded as immobile. The immobility time-duration was considered in the last 5 min.
Morris water maze test (MWM)
Cognitive impairment in the UL122 mice was determined using the MWM test. Water maze equipment included circular tanks filled with running water and covered with white plastic foam. The pool was divided into four quadrants, and was filled to a depth of 30 cm with water with an invisible platform in one quadrant center. Parameters measured were average speed, platform crossings and quadrant time (%) in the objective quadrant without the platform on the last day.
Novel object recognition test (NOR)
We investigated the exploration of familiar and novel objects using the NOR, which assesses cognitive ability. Briefly, mice were adapted to the empty box for 5 min each day over an adaptation period of 2 days. Mice were placed in the box for 5 min during the training stage. During testing sessions, one familiar object was alternated with one novel object of similar size in the same position as in the training phase, and exploration was allowed for 5 min. The recognition index (RI) was represented as the time exploring the novel objects divided by the total time spent exploring both objects.
Microarray analysis
mRNA samples were isolated from the hippocampus of UL122 and control mice. Three biological replicates were utilized in this experiment. Microarray analysis was conducted using the Illumina Hiseq platform, and differentially expressed genes were identified by comparing the expression profiles of the two sets of mice. The genes related to neurogenesis were further investigated using high-throughput quantitative polymerase chain reaction (qPCR).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using an RNA isolation kit (TIANGEN) according to the manufacturer’s instruction. The cDNA was synthesized using a reverse transcription kit (Roche). Five microliters of reverse transcripts were added to a 15 µl PCR mixture (FastStart Essential DNA Green Master) for 40 cycles using a Bio-Rad instrument. The levels of FGF2, NeuN, GFAP, and β-actin mRNA were measured using the SYBR Green I assay. The primers were as follows: 5’-AAGCGGCTCTACTGCAAGAACG-3’ and 5’-CAGCCGTCCATCTTCCTTCATAGC-3’ (FGF2, 186 bp); 5’-TGGAGCGGTCGTGTATCAGGATG-3’ and 5’-TCAGCAGCGGCATAGACTCTACC-3’ (NeuN, 138 bp); 5’-CCGCCAAGCCAAGCACGAAG-3’ and 5’-CTCCTCCTCCAGCCGAGCAAG-3’ (GFAP, 178 bp).
Western blot
The hippocampus was harvested for protein extraction. All protein extracts were boiled for 5 min and separated on SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes. The membranes were blocked with blocking buffer. The following antibodies were incubated with the membranes: rabbit anti-mouse FGF2 (Bioss, 1:3000), rabbit anti-mouse GFAP (Bioss, 1:3000), rabbit anti-mouse NeuN (Abcam, 1:5000), rabbit anti-mouse SYP (Abcam, 1:5000) and rabbit anti-mouse PSD-95 (Abcam, 1:5000). Horseradish peroxidase-conjugated goat anti-rabbit antibodies were used as secondary antibodies, and the immunoblots were visualized using ECL reagents.
Hematoxylin-eosin (HE) staining
Hippocampal neuron morphology was observed using H&E staining. Approximately 3-μm paraffin sections the hippocampus tissues were stained with hematoxylin solution for 5 min and eosin solution for 3 min in sequence. Histopathological changes were examined using a light microscope (Olympus, Japan).
Immunohistochemistry
First, 3-μm hippocampus sections were placed on charged slides, baked at 70°C for an hour, then deparaffinized, rehydrated, and placed in boiling water for two minutes pretreated for antigen retrieval with sodium citrate buffer. Slides were cooled to room temperature, then washed with double distilled water and PBS for 15 min. Three percent hydrogen peroxide was used to inactivate endogenous catalase. Then, sections were incubated for one hour with primary antibodies (anti-FGF2, anti-NeuN and anti-GFAP antibodies diluted at 1:3000, Bioss; anti-IE2 antibody diluted at 1:100, Millipore, USA). Sections were incubated with secondary antibodies for 40 min. The tissue sections were then exposed to DAB for 1 min and hematoxylin for 2 seconds, and observed after placement of neutral gum seal.
Statistical analysis
Statistical analyses were performed using Prism 9.0 (GraphPad, La Jolla, CA, US). Study results were expressed as means ± standard deviations. Statistics from animal behavior test, qRT-PCR, and western blot were analyzed using Student’s-t test. P<0.05 was considered significant. Statistical significance was also taken as *P<0.05, **P<0.01 and ***P<0.001.
Results
Identification of the UL122 mice
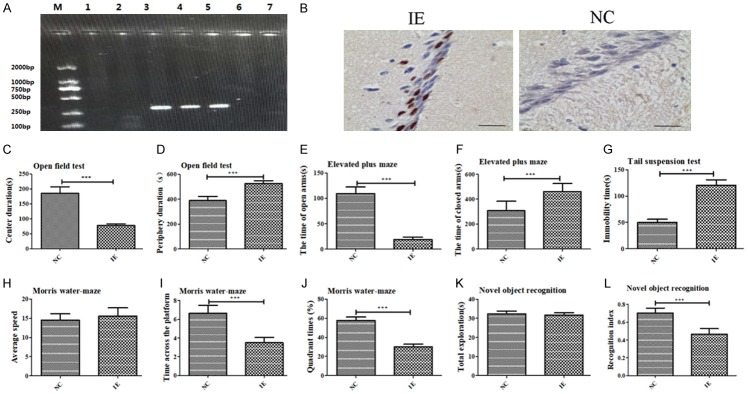
PCR showed that we extracted UL122 genes from mice (Figure 1A). The mice were separated into experimental and control groups based on the presence or absence of UL122. UL122-positive mice were the experimental group (IE) and the UL122-negative mice served as controls (NC). Expression of IE2 in mice hippocampus was observed using immunohistochemistry; IE2 expression in the experimental group was strongly positive, but was negative in the control group (Figure 1B).
Figure 1.
HCMV-encoded IE2 induces anxiety-depressive and cognitive impairment in UL122 genetically-modified mice. A. Identification of positive (Lanes 1-2) and negative mice (Lanes 3-5) using PCR (lanes 6-7 are water). B. Compared with the control group (NC), nuclear staining was stronger positive in the CA1 of the experimental group (IE). Bar: 400 µm. C. Time spent in the center regions of the open field. D. Time spent in the periphery of the open field. E. Time spent in the open arms in elevated plus-maze test. F. Relative time spent in the closed arms in the elevated plus-maze test. G. Immobility time of the experiment group was significantly longer on the tail suspension test. H. There was no significant difference in average speed in the MWM between groups. I. The number of platform crossings was significantly lower in the IE group. J. The IE group spent less amount time in the objective quadrant. K. There was no significant difference in overall exploration in NOR. L. RI in the experimental group was lower. Statistical significance was set as ***P<0.001.
Anxiety-like behaviors were increased in UL122 mice
We performed open field and elevated plus-maze tests to measure anxiety-like behavior. IE mice spent significantly less time in the central regions of the open field (P<0.001; Figure 1C) and significantly more time motion in the periphery (P<0.001; Figure 1D) than did NC mice. Results of the elevated plus-maze test were consistent with those of the open field test, with the IE group showing similar anxiety behaviors. The IE group tended to spend less time in the open arms (P<0.001; Figure 1E) and longer periods in the closed arms than did NC group mice (P<0.001; Figure 1F).
Depressive-like behaviors were increased in UL122 mice
We used the tail suspension test to assess depression-like behavior. Traditionally, immobility is perceived as abandonment behavior that can be expressed as depression in the test. We observed that the IE group showed substantially more such behavior than did the NC group (P<0.001; Figure 1G).
Lowered cognitive ability in UL122 mice
To measure cognitive-related behavior of mice, we used the Morris water maze test and the novel object recognition test. On MWM test, the difference in average speed in the IE group did not differ from that of the NC group (P>0.05; Figure 1H). The number of platform crossings was significantly lower in the IE group than in the NC group (P<0.001; Figure 1I). The IE group spent less amount time in the objective quadrant than did the NC group (P<0.001; Figure 1J). As in the NOR, there were no differences in terms of overall exploration, suggesting no spontaneous bias for an object relative to NC mice (P>0.05; Figure 1K). The recognition index in the IE group was significantly lower than that of the NC during the test period (P<0.001) (Figure 1L). This suggests that IE2 impaired cognitive ability to some degree.
IE2 promoted hippocampal neuron damage in UL122 mice
The damage of hippocampal neurons in the UL122 transgenic mice was assessed using light microscopy (Figure 2). HCMV-encoded IE2 expression gave rise to disorganization of hippocampal neurons in the IE group. Some neurons were observed to be damaged, characterized by neuron shrinkage with wider intercellular spaces than in NC group. Compared with the NC group, neuronal damage in the IE group was substantial, suggesting that IE2 might have caused neurodegenerative changes in CA1, CA3, and DG of the hippocampus (Figure 2A and 2B).
Figure 2.
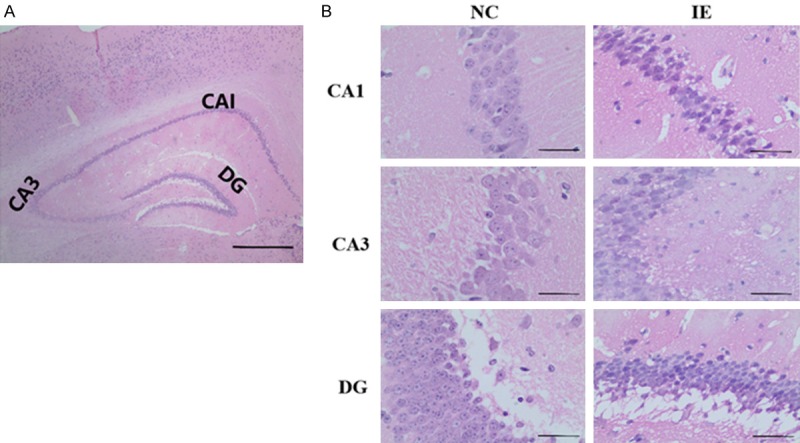
Effect of IE2 on morphological changes in the hippocampal CA1, CA3 and DG region of UL122 transgenic mice. A. The histologic zonal structure of hippocampal. B. Compared with the control group, neuron morphology and arrangement in the experimental group show substantial abnormalities. Bar: 400 μm.
IE2 down regulates FGF2 expression in the hippocampus of UL122 mice
Microarray analysis data showed that IE2 inhibited neurogenesis-related genes expressed in transgenic mouse hippocampus. There was decreased expression of neurogenesis-related genes, including Fgf2, Foxo3, Cdkl3, Apoe, Cpeb3, Etv1, Ilk and Mef2c (Figure 3A). This was further confirmed using qPCR (Figure 3B). In particular, Fgf2 is closely involved with depression, anxiety, and cognitive impairment. Quantitative and semiquantitative PCR were used to investigate mRNA expression of Fgf2 (P<0.001; Figure 3B and 3C). Western blot and immunohistochemistry were used to measure FGF2 protein expression. We found that FGF2 expression was lower in the IE group than in the NC group (P<0.001; Figure 3D and 3E).
Figure 3.
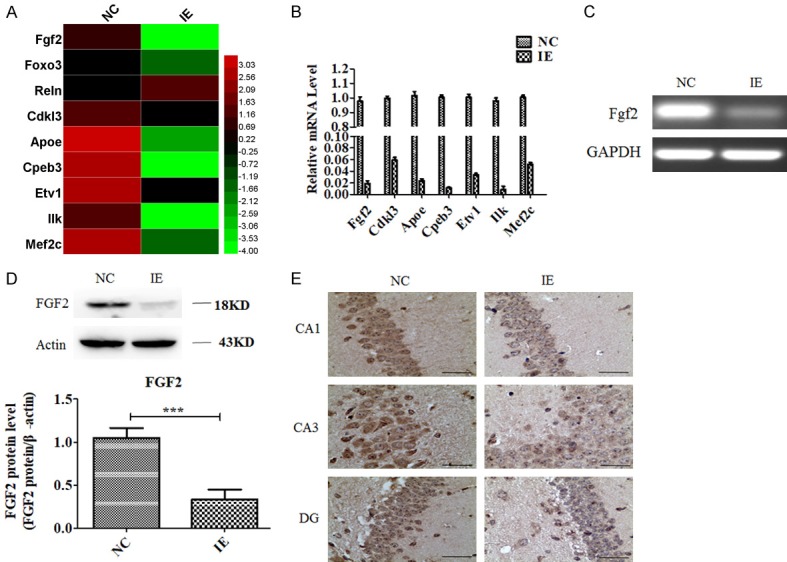
IE2 down-regulates FGF2 expression in UL122 transgenic mice hippocampus. (A-C) Microarray analysis data (A), real-time qPCR (B) and semiquantitative PCR (C) showing that IE2 inhibits neurogenesis-related gene expression. (D and E) Immunohistochemistry (D) and western blot (E) showing that IE2 inhibits the expression of FGF2 protein. Bar: 400 μm. ***P<0.001.
Decreased NeuN and GFAP expression in UL122 mice
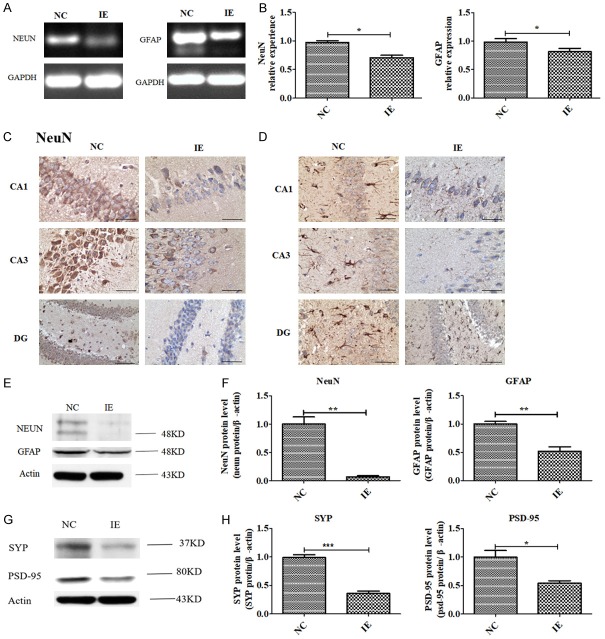
NeuN (mature neuronal marker) and GFAP (astrocyte marker) modulate synaptic plasticity; neurons and astrocytes play important roles in depression, anxiety, and cognitive impairment [11-14]. We found significantly lower levels of mRNA expression for NeuN and GFAP in the CA1, CA3 and DG of the hippocampus of the IE group than that of the NC group (P<0.05; Figure 4A and 4B). We also measured protein levels of NeuN and GFAP using immunohistochemistry (Figure 4C and 4D) and western blot (P<0.01; Figure 4E and 4F). We found that levels of NeuN and GFAP in the CA1, CA3 and DG of the hippocampus were significantly lower in the IE group than in the NC group.
Figure 4.
Decreased the level of NEUN, GFAP, SYP and PSD-95 expression in UL122 transgenic mice. (A and B) Semiquantitative PCR (A) and qPCR (B) showing that IE2 inhibits the expression of NeuN and GFAP. Immunohistochemistry (C and D) and western blot (E and F) showing that IE2 inhibits the expression of NEUN and GFAP protein. (G and H) SYP and PSD-95 protein levels in hippocampus. *P<0.05, **P<0.01, ***P<0.001.
Decreased SYP and PSD-95 proteins in hippocampus of UL122 mice
It is reported that NeuN and GFAP have significant influence on neurodevelopment and synaptic plasticity in the hippocampus implicated in cognition and emotion [15,16]. To further explore effect of decreased NeuN and GFAP expression on synaptic plasticity in UL122 mice, we measured the expression of synaptophysin (SYP) and postsynaptic density 95 (PSD-95) in the hippocampus. Western blotting was used to assess the expression of SYP and PSD-95. We found that expression levels of SYP and PSD-95 protein were significantly lower in the hippocampus of IE mice (Figure 4G and 4H), suggesting NeuN and GFAP modulate synaptic plasticity.
Discussion
To the best of our knowledge, this study is the first to describe cognitive and emotion-related behaviors in UL122 genetically-modified mice. Using behavioral tests, we showed greater levels of anxiety-like behaviors of UL122 genetically-modified mice in open field and elevated plus-maze tests. UL122 mice also demonstrated more depressive-like behaviors in tail-suspension test. Furthermore, UL122 mice demonstrated reduced cognitive capacity in the Morris water maze and novel object recognition test. These data suggest changes in cognition and mood associated with HCMV-encoded IE2.
The IE2 encoded by UL122 in HCMV has been extensively studied because it plays an important role in viral replication and has been implicated in the pathogenesis of some diseases [17]. Furthermore, the biologic role of IE2 in the nervous system has attracted more attention. IE2 was associated with brain dysfunction, mental retardation, visual and hearing disorders, seizures, and epilepsy [18]. High levels of HCMV seropositivity correlated with cognitive decline in patients with Alzheimer’s disease [19]. Subsequently, our data of MWM and NOR tests suggested that cognitive capacity was lower in UL122 mice. People with higher CMV-specific antibody titers were more likely to be depressed and anxious [8]. We observed similar phenomena in OF and TST. Taken together, these findings support the notion that IE2 genes are important in emotional and cognitive disorders.
Emotional and cognitive abilities are modulated by synaptic plasticity, reflecting synaptic function. NeuN (mature neuronal marker) and GFAP (astrocyte marker) have significant influence on neurodevelopment, and synaptic plasticity in the hippocampus that is implicated in modulation of cognition and emotion [15,16]. To further determine whether synaptic function was affected by decreased NeuN and GFAP expression in UL122 mice, we measured expression levels of SYP and PSD-95 that are synaptic plasticity-associated proteins [20]. The low levels of expression of SYP and PSD-95 reflected decreased synaptic plasticity. The decreased synaptic plasticity of UL122 genetically-modified mice was consistent with the results of the animal behavior tests. These findings suggest that decreased NeuN and GFAP had a profound negative impact on the synaptic function.
Among 22 FGF family members, several lines of evidence show that expression of FGF2 protein plays a key role in the development of mood disorders and cognitive impairments by regulating neurogenesis and synaptic plasticity during development and adulthood [21,22]. Lower levels of FGF2 mRNA were detected in brains of human cadavers who had suffered from major depressive disorders than in controls [23]. Consequently, several possible applications of FGF2 have been proposed as therapies for neurodegenerative conditions, including Alzheimer’s disease and Parkinson’s disease [24]. Several lines of evidence suggest that neurogenesis and synaptic plasticity may be important for memory and mood [25]. FGF2 has also been associated with NEUN and GFAP expression. Both in neuronal and in glial cells in the CNS, FGF2 is expressed abundantly. FGF2 treatment increases neurogenesis, survival, and proliferation of neurons and astrocytes in several studies. We found that IE2 significantly down-regulated the expression of NeuN, GFAP and FGF2 in UL122 mice. Decreased FGF2 was keeping with the downregulation of NeuN, GFAP synchronously.
In summary, we demonstrated a key role of the HCMV immediate-early 2 (IE2) protein in HCMV-caused cognitive and anxiety-depressive behaviors. In UL122 mice, because of synchronously decreased expression levels of NeuN, GFAP and FGF2, we propose that IE2-FGF2-mediated NeuN and GFAP may represent a novel mechanism underlying the mechanisms of HCMV-induced depression, anxiety, and cognitive impairment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NO. 81471958).
Disclosure of conflict of interest
None.
References
- 1.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68:1058–1064. doi: 10.1001/archgenpsychiatry.2011.113. [DOI] [PubMed] [Google Scholar]
- 2.Esposito M, Sherr GL. Epigenetic modifications in Alzheimer’s neuropathology and therapeutics. Front Neurosci. 2019;13:476. doi: 10.3389/fnins.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao J, Li K, Du L, Yin H, Tan X, Yang Z. Deletion of asparagine endopeptidase reduces anxiety- and depressive-like behaviors and improves abilities of spatial cognition in mice. Brain Res Bull. 2018;142:147–155. doi: 10.1016/j.brainresbull.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Clement M, Humphreys IR. Cytokine-mediated induction and regulation of tissue damage during cytomegalovirus infection. Front Immunol. 2019;10:78. doi: 10.3389/fimmu.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavuljica I, Kvestak D, Huszthy PC, Kosmac K, Britt WJ, Jonjic S. Immunobiology of congenital cytomegalovirus infection of the central nervous system-the murine cytomegalovirus model. Cell Mol Immunol. 2015;12:180–191. doi: 10.1038/cmi.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chvatalova V, Sebankova B, Hrbackova H, Turecek P, Flegr J. Differences in cognitive functions between cytomegalovirus-infected and cytomegalovirus-free university students: a case control study. Sci Rep. 2018;8:5322. doi: 10.1038/s41598-018-23637-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wu D, Yang L, Xu XY, Zhang GC, Bu XS, Ruan D, Tang JL. Effects of congenital HCMV infection on synaptic plasticity in dentate gyrus (DG) of rat hippocampus. Brain Res. 2011;1389:27–34. doi: 10.1016/j.brainres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Phillips AC, Carroll D, Khan N, Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun. 2008;22:52–55. doi: 10.1016/j.bbi.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Hagemeier C, Walker S, Caswell R, Kouzarides T, Sinclair J. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J Virol. 1992;66:4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K, Murtuza B, Suzuki N, Khan M, Kaneda Y, Yacoub MH. Human cytomegalovirus immediate-early protein IE2-86, but not IE1-72, causes graft coronary arteriopathy in the transplanted rat heart. Circulation. 2002;106(Suppl 1):I158–162. [PubMed] [Google Scholar]
- 11.Yu M, Yang D, Wang M, Wei X, Li W. Early stage of diffusional kurtosis imaging and dynamic contrast-enhanced magnetic resonance imaging correlated with long-term neurocognitive function after experimental traumatic brain injury. Neurosci Lett. 2019;705:206–211. doi: 10.1016/j.neulet.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, Davies J, Doldan NG, Arao S, Ferdousi F, Szele FG, Isoda H. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging (Albany NY) 2019;11:401–422. doi: 10.18632/aging.101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zappa Villar MF, Lopez Hanotte J, Falomir Lockhart E, Tripodi LS, Morel GR, Reggiani PC. Intracerebroventricular streptozotocin induces impaired Barnes maze spatial memory and reduces astrocyte branching in the CA1 and CA3 hippocampal regions. J Neural Transm (Vienna) 2018;125:1787–1803. doi: 10.1007/s00702-018-1928-7. [DOI] [PubMed] [Google Scholar]
- 14.Iwata M, Shirayama Y, Ishida H, Hazama GI, Nakagome K. Hippocampal astrocytes are necessary for antidepressant treatment of learned helplessness rats. Hippocampus. 2011;21:877–884. doi: 10.1002/hipo.20803. [DOI] [PubMed] [Google Scholar]
- 15.Lin YS, Wang HY, Huang DF, Hsieh PF, Lin MY, Chou CH, Wu IJ, Huang GJ, Gau SS, Huang HS. Neuronal splicing regulator RBFOX3 (NeuN) regulates adult hippocampal neurogenesis and synaptogenesis. PLoS One. 2016;11:e0164164. doi: 10.1371/journal.pone.0164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Scholz M, Doerr HW, Cinatl J. Inhibition of cytomegalovirus immediate early gene expression: a therapeutic option? Antiviral Res. 2001;49:129–145. doi: 10.1016/s0166-3542(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 18.Han D, Byun SH, Kim J, Kwon M, Pleasure SJ, Ahn JH, Yoon K. Human cytomegalovirus IE2 protein disturbs brain development by the dysregulation of neural stem cell maintenance and the polarization of migrating neurons. J Virol. 2017:91. doi: 10.1128/JVI.00799-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasko I, Knaus G, Weiss E, Kemmler G, Winkler C, Falkensammer G, Griesmacher A, Wurzner R, Marksteiner J, Fuchs D. Cognitive deterioration in Alzheimer’s disease is accompanied by increase of plasma neopterin. J Psychiatr Res. 2007;41:694–701. doi: 10.1016/j.jpsychires.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Liao Y, Li T, Cui Y, Wang G, Zhao F, Jin Y. Alterations of synaptic proteins in the hippocampus of mouse offspring induced by developmental lead exposure. Mol Neurobiol. 2016;53:6786–6798. doi: 10.1007/s12035-015-9597-0. [DOI] [PubMed] [Google Scholar]
- 21.Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ. Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist. 2013;19:479–494. doi: 10.1177/1073858412472399. [DOI] [PubMed] [Google Scholar]
- 22.Salmaso N, Stevens HE, McNeill J, ElSayed M, Ren Q, Maragnoli ME, Schwartz ML, Tomasi S, Sapolsky RM, Duman R, Vaccarino FM. Fibroblast growth factor 2 modulates hypothalamic pituitary axis activity and anxiety behavior through glucocorticoid receptors. Biol Psychiatry. 2016;80:479–489. doi: 10.1016/j.biopsych.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer’s disease and has therapeutic implications for neurocognitive disorders. Proc Natl Acad Sci U S A. 2011;108:E1339–1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A, Yau SY, Machado S, Wang P, Yuan TF, So KF. Enhancement of hippocampal plasticity by physical exercise as a polypill for stress and depression: a review. CNS Neurol Disord Drug Targets. 2019;18:294–306. doi: 10.2174/1871527318666190308102804. [DOI] [PubMed] [Google Scholar]