Fig. 5.
Downstream process overview and results.
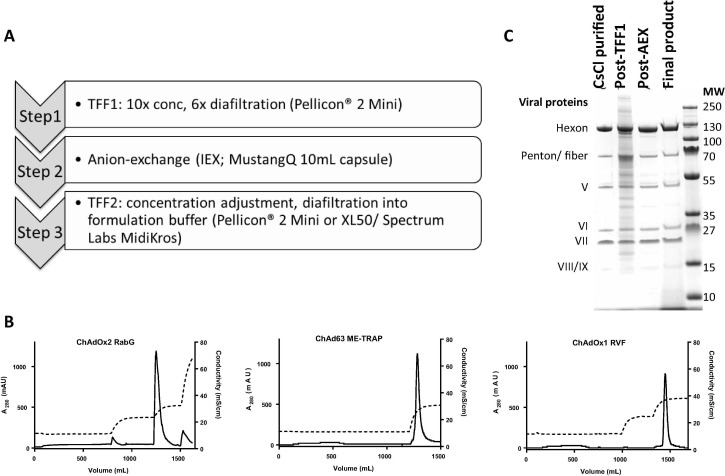
Panel A shows an overview of the downstream process steps.
Panel B shows conductivity and A280 traces obtained for at-scale chromatography for each of the three vaccines, as indicated in captions for each plot. In each case, loading occurs over the first ∼ 600 mL (a degree of A280 deflection from impurity flow-through is visible) and the largest A280 peak represents the eluted virus which was collected. Note for ChAdOx2 RabG that the pre-elution wash and the post-elution high-salt wash produce relatively small A280 peaks (predominantly impurity, with some virus, as assessed by SDS-PAGE); this data was obtained using UV and conductivity sensors of the Akta Purifier. For ChAd63 and ChAdOx1, data was obtained using the complete single-use chromatography rig (i.e. with single-use UV and conductivity sensors [Pendotech]) and no post-elution wash was performed. For ChAd63, due to the lower conductivity at which this vaccine eluted, no pre-elution wash was used.
Panel C shows samples from each stage of the downstream process on a Coomassie-stained SDS-PAGE gel. Samples shown are from a single process run with the ChAdOx2 RabG vaccine, but are typical of runs with all three investigated vaccines.