Abstract
Endometriosis is a chronic inflammatory and estrogen-dependent disease that causes pain and infertility in reproductive-aged women. Due to the delay in diagnosis, there is a pressing need for accurate biomarkers. Detection of serum noncoding RNA molecules such as microRNAs (miRNAs) shows promise as a noninvasive diagnostic strategy; we previously identified miRNAs that are highly sensitive and specific biomarkers for the disease. In this study, we investigate the expression of these miRNAs in a nonhuman primate model of endometriosis. As part of a pilot study evaluating simvastatin for the treatment of endometriosis, the disease was induced in 16 baboons by induction laparoscopy and the animals were divided into 2 groups. One group was treated with simvastatin for 90 days, while the second group received vehicle only. Endometriosis was evaluated after 3 months by laparoscopy. Serum samples were analyzed for 9 circulating miRNAs using quantitative real time-polymerase chain reaction, focusing on the miRNAs we found to be dysregulated in human endometriosis. In the simvastatin-treated endometriosis group, levels of miR-150-5p and miR-451a were decreased, while miR-3613-5p levels were increased compared to the untreated endometriosis group. The changes in circulating miRNA expression patterns parallel our previous results in human patients and show that specific miRNAs correlate with endometriosis severity and reverted toward control expression levels after simvastatin treatment. This is the first report showing serum miRNA expression normalized in response to endometriosis treatment, supporting the potential for this class of biomarkers to be used both to diagnose endometriosis and to monitor its progression and response to therapy.
Keywords: baboons, endometriosis, statins, simvastatin, miRNAs, biomarker
Introduction
Endometriosis is a chronic, estrogen-dependent inflammatory disease that adversely affects life quality and productivity.1,2 It is characterized by the presence and growth of endometrial glands and stroma outside the uterus and affects between 6% and 10% of all women of reproductive age.1,2 The etiology and pathogenesis of the disease are not fully defined. Various theories such as retrograde menstruation, lymph vascular metastasis, ectopic differentiation of stem cells, dysregulated inflammatory responses, and exposure to environmental factors have been proposed.3–6 Clarifying the mechanisms of endometriosis pathogenesis may result in new therapies, by identifying new molecular targets for pharmacological mediation.7 The majority of the current drugs are designed to alter sex steroids, and ongoing research is focused on developing new drugs that target the molecular mechanisms involved in the pathogenic cascade of the disease.8 Previously, we identified statins as a class of drugs with efficacy in the treatment of endometriosis.8–11 Here, we assessed the serum from our statin trial in baboons to determine whether the microRNA (miRNA) biomarkers that we reported as altered in human endometriosis would respond to treatment in parallel with the disease.
The baboon model of induced endometriosis is well suited for use in research to better understand the development of the disease and is considered an ideal preclinical model to for evaluating effects of medical treatments.12 Like human females, female baboons also menstruate and can develop spontaneous as well as induced endometriosis. The model system allows researchers to control many experimental variables that could not be manipulated in human studies and makes it possible to observe the progression of lesions by repeated laparoscopic evaluation over short time intervals. In humans, serial laparoscopies are not practical or ethical, with surgery performed only when medically necessary. In animals, the timing of induction of the disease is controlled, and endometrial lesion development and the posttreatment reduction in lesions can all be observed laparoscopically.13 Measuring markers of the disease in control groups of animals before and after induced endometriosis is also feasible in baboon studies, which would be difficult in human subjects. As such, the baboon model may well be the best animal model of endometriosis; it closely approximates the human condition and can be used for elucidation of endometriosis pathophysiology.12,14
In a recent pilot study, our group assessed the efficacy of the statin, simvastatin, on endometriosis in baboons.9 Statins represent a potential alternative approach for treating endometriosis in women for whom hormonal treatments are ineffective or cause undesirable side effects. These drugs inhibit synthesis of both cholesterol and isoprenoid intermediates, causing a multitude of downstream effects on cells including a reduction in inflammatory activity and oxidative stress.8 Independent of their effect on cholesterol, statins inhibit cell proliferation and concomitantly increase cell apoptosis in human endometrial cell cultures.15–17 Statins inhibit endometriotic stromal cell proliferation by inhibiting Rho activation and suppress the attachment of these cells to collagen fibers preventing fibrosis.18 In vitro and in rodent, statins arrest neoangiogenesis in endometriotic cells through reduction in VEGF gene transcription19–21 and reduce endometrial cell adhesiveness and invasiveness due to a decrease in cluster of differentiation (CD44) metalloproteinase (MMP)s and expression in association with an increase in tissue inhibitor of metalloproteinases 2 (TIMP-2) activity.10,20,22 Statins also inhibit the gene expression of pro-inflammatory markers such as cyclooxygenase-2 (COX2), vascular endothelial growth factor (VEGF), receptor for advanced glycation endproducts (RAGE), and extracellular RAGE-binding protein (EN-RAGE), monocyte chemoattractant protein-1 (MCP-1), while increasing anti-inflammatory gene transcription markers like peroxisome proliferator-activated receptor gamma (PPAR-γ), liver X receptor alpha (LXR-α), and superoxide dismutase (SOD).11,19,20 Because of these properties, statins have been used in the treatment of pelvic pain in patients affected by endometriosis.23 Our recent study tested the effects of simvastatin treatment for the first time in a primate model of induced endometriosis, and reported a decrease in the volume of active endometriotic lesions, along with decreased expression of key markers of cell proliferation, inflammation, and oxidative stress.9 Building on that pilot investigation, here we expand our analysis to investigate changes in expression levels of a class of regulatory biomarkers in serum: circulating miRNAs.9,24
MicroRNAs are a class of small (21-24 nucleotides), nonprotein coding conserved RNAs in the cell that regulate gene expression by controlling translation of target mRNAs.25 Cells secrete miRNAs either actively or passively through cell lysis. They can enter the circulation where they are stable without significant degradation, due to their small size and encapsulation in microvesicles or association with protein complexes. The spectrum of miRNAs in circulation reflects cell state as well as physiological and pathological state of the organism.24,26 Circulating miRNAs exhibit specific characteristics that make them strong biomarker candidates for the diagnosis of various diseases.27–29 Cell-free miRNAs are stably present in several body fluids, including blood serum and plasma, urine, and saliva.29 The expression of pathologic miRNAs has been shown in gynecological diseases including endometriosis and related infertility.27,30–32
Several reports, including those from our laboratory, demonstrated differentially expressed miRNA levels in serum of patients with endometriosis; levels of several miRNAs yielded biomarkers with high diagnostic power.27,29,31,33 In a previous study,28 we compared miRNA expression in the serum of women with and without endometriosis using microarray analysis and confirmed our results with quantitative real time-PCR (qRT-PCR). In that study, miR-125b-5p, miR-150-5p, miR-342-3p, miR-143-3p, miR-500a-3p, miR-451a, and miR-18-5p were significantly increased while miR-6755-3p and miR-3613-5p were significantly decreased.31 A recent study by Nothnick and colleagues also reported elevated levels of serum miR-451a in patients with endometriosis and found similar upregulation of miR-451a in baboon serum.34 In the current study, we investigate the expression of these endometriosis-associated circulating miRNAs in a baboon endometriosis model and their response to treatment with simvastatin.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Scientific Evaluation and Review Committee and Animal Care and Use Committee of both Yale University and the Institute of Primate Research. Healthy adult female baboons (n = 16), Papio anubis, were taken from the wild and kept in quarantine for 3 months. Animals were screened for tuberculosis, simian immunodeficiency virus, and simian T-lymphotropic virus-1, and all tested negative for these diseases. During the initial laparoscopy, the pelvis of each animal was examined, and those lacking any evidence of preexisting spontaneous endometriosis were selected. All procedures in the study were carried out according to the Institute of Primate Research standard operating procedures and described protocols.12 Complete details of the experimental procedures were as described in Taylor et al.9
Briefly, endometriosis was induced by laparoscopy, by seeding in the peritoneal cavity of female baboons with menstrual endometrium (n = 16).9,12,14 Menstrual endometrium was obtained using a Unimar Pipelle (CooperSurgical Inc, Shelton, Connecticut) before laparoscopy. Under laparoscopic guidance, approximately 1 g of menstrual tissue and fluid was delivered from the Pipelle at 4 sites: the pouch of Douglas, the uterine fundus, the cul-de-sac, and the ovaries. During the induction laparoscopy, the pelvic cavity of each animal was examined for the presence of spontaneous endometriosis, and one animal in the simvastatin group had a single black lesion (1.5 × 1.5 × 0.5 mm). All 16 baboons with seeding of endometrial tissue were randomized into 2 groups, by a laboratory technician opening a sealed envelope specifying the assignment into control or treatment group. Beginning the day after induction, the treatment group (n = 8) was treated daily with simvastatin (20 mg/d) added to the banana meal diet for 90 days, and the control group (n = 8) received vehicle diet alone. At 3 months, a laparoscopy was performed to evaluate the extent of the disease in both groups. The peritoneal cavity of each animal was inspected thoroughly for the presence of lesions, and the observations were documented by videotaping. Endometriotic lesions and adhesions were counted, and each lesion was measured in 3 dimensions. Types of lesions included blue-black, white, red, orange-red; adhesions included filmy and dense. Further detail on each type of lesion and a breakdown of volume analysis by lesion type can be found in Taylor et al.9 To calculate lesion volume, length × width × depth was calculated (mm3).
Sample Collection and RNA Extraction
Blood was collected in sterile tubes from each baboon just before performing the 3-month laparoscopy (day 91), and centrifuged immediately to collect serum, which was frozen at −80°C for the analysis of miRNAs. Total RNA was extracted from 200 μL of serum using the Qiagen RNA Isolation Kit (Qiagen, Valencia, California) according to the manufacturer’s specifications and was eluted with 50 μL of nuclease-free water. The yield of RNA was determined using a NanoDrop ND-2000 spectrophotometer (Nanodrop Technologies, Wilmington, Delaware).
Quantitative Real-time Polymerase Chain Reaction for miRNAs
Total RNA (25 ng) from each sample was reverse transcribed using an Invitrogen NCode miRNA First-Strand cDNA Synthesis MIRC-50 kit (Life Technologies, Carlsbad, California) following the manufacturer’s instructions. Levels of 9 miRNAs, based on the miRNAs previously identified in patients with human endometriosis,31 were quantified by qRT-PCR using the iQ SYBR Green supermix kit (Bio-Rad Laboratories, Hercules, California), with the specific forward primers (Table 1) for different miRNAs and the universal reverse primer complementary to the anchor primer. The thermal cycling conditions were initiated by uracil-N-glycosylase activation at 50°C for 2 minutes and initial denaturation at 95°C for 15 minutes, followed by 50 cycles at 95°C for 15 seconds and annealing at 59°C for 50 seconds. Threshold cycle and melting curves were acquired by using the quantitation and melting curve program of the Bio-Rad iCycler iQsystem (Bio-Rad Laboratories). Anchor reverse-transcription primer was used as the template for negative control, and U6 small nuclear RNA was used as an endogenous control to determine relative miRNA expression.31 The relative miRNA levels were determined using the comparative cycle threshold (Ct) method (2− Δ CT). All experiments were carried out 3 times, each in triplicate and nuclease-free water was used as a negative control replacing the cDNA template. Primers for miRNAs and the U6 genes were obtained from the W.M. Keck Oligonucleotide Synthesis Facility (Yale University, New Haven, Connecticut).
Table 1.
Primer Sequences Used for qRT-PCR of miRNAs.
| miRNA | Primer | Sequence |
|---|---|---|
| miR-125b-5p | Forward | UCCCUGAGACCCUAACUUGUGA |
| miR-150-5p | Forward | UCUCCCAACCCUUGUACCAGUG |
| miR-342-3p | Forward | UCUCACACAGAAAUCGCACCCGU |
| miR-145-5p | Forward | GUCCAGUUUUCCCAGGAAUCCCU |
| miR-143-3p | Forward | UGAGAUGAAGCACUGUAGCUC |
| miR-500a-3p | Forward | AUGCACCUGGGCAAGGAUUCUG |
| miR-451a | Forward | AAACCGUUACCAUUACUGAGUU |
| miR-6755-3p | Forward | UGUUGUCAUGUUUUUUCCCUAG |
| miR-3613-5p | Forward | UGUUGUACUUUUUUUUUUGUUC |
| U6 | Forward | CTCGCTTCGGCAGCACA |
Abbreviations: miRNA, microRNA; qRT-PCR, quantitative real time-polymerase chain reaction.
Statistical Analysis
Mean total disease volume (including lesions and adhesions) between the groups (simvastatin-treated vs untreated) was compared using an unpaired t test with Welch correction for unequal variances. The expression levels of serum miRNAs between the groups were also compared using an unpaired t test with Welch correction. Statistical analysis was performed using SPSS 16.0 (SPSS Inc, Chicago, Illinois) or Graphpad Prism, Version 7. P < .05 was considered statistically significant, and the data were expressed as mean ± SEM.
Results
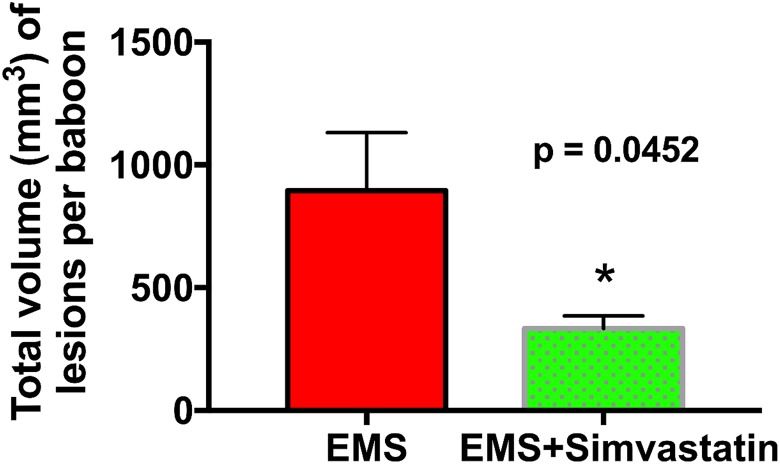
Endometriosis was induced in 16 baboons, which were subsequently randomized into 2 groups, one group (n = 8) which received simvastatin as an endometriosis therapy, and a control group (n = 8) to which no active treatment was administered.9 As reported previously, simvastatin treatment caused a significant decrease in the combined volume of red, orange-red, and white lesions.9 In this subset of active lesions, the average lesion volume in baboons the simvastatin-treated group was 26.4 ± 6.9 mm3 (average ± SD), approximately 78% lower than 117.4 ± 64.6 mm3 in untreated animals.9 We performed our own independent analysis of the total disease volume per baboon and report the combined volume of all colors and types of lesions as well as pelvic adhesions. Our analysis of total disease volume indicated a decrease in average volume of roughly 65%, from 896 ± 667 mm3 in untreated animals to 301 ± 178 mm3 in simvastatin-treated baboons (Figure 1). The total number of lesions per baboon (analyzed previously and independently confirmed here) was not significantly different between groups.9
Figure 1.
Average total volume of disease (combined volume of lesions and adhesions) per baboon in simvastatin-treated baboons (n = 8) versus controls (n = 8). Includes all types of lesions and adhesions (see methods). Data are expressed as mean ± SEM. *P < .05, using an unpaired t test with Welch correction. Endometriosis indicates endometriosis.
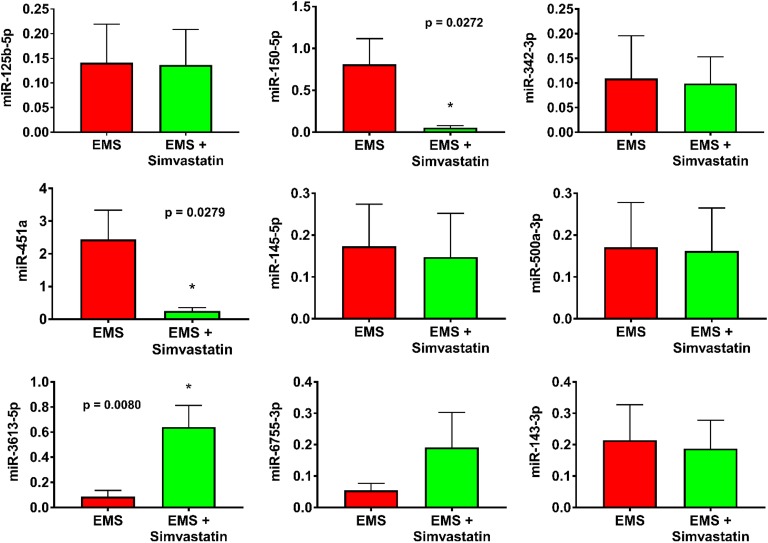
Differential expression of miRNAs has been reported in endometriosis, and these expression level changes may be used as biomarkers of endometriosis. We previously identified circulating miRNAs that were significantly changed in the serum of patients with endometriosis, and here we measured expression of 9 of these miRNAs (miR-125b-5p, miR-150-5p, miR-342-3p, miR-143-3p, miR-145-5p, miR-500a-3p, miR-451a, miR-6755-3p, and miR-3613-5p) to compare levels in the serum of baboons with untreated endometriosis versus endometriotic baboons treated with simvastatin (endometriosis + simvastatin). Data presented in Figure 2 summarize the expression levels of the 9 miRNAs we tested, normalized to U6 small nuclear RNA levels. Serum levels of miR-150-5p and miR-451a were decreased significantly in the simvastatin-treated endometriosis group of baboons compared to the untreated endometriosis group. The decrease in circulating miRNA level was 93% (P = .04) for miR-150-5p, and 90% (P = .04) for miR-451a. In contrast, miR-3613-5p levels were increased by roughly 7.5-fold (P = .01) in the simvastatin-treated group relative to endometriosis group without treatment. No statistically significant changes were measured in the levels of other miRNAs.
Figure 2.
Differential expression of circulating microRNAs (miRNAs) in response to simvastatin treatment of endometriosis in baboons. Serum was collected from simvastatin-treated baboons with endometriosis as well as untreated controls at 3-month postinduction of endometriosis. Expression levels were determined by quantitative real time-polymerase chain reaction (qRT-PCR) and normalized to U6 expression levels. Error bars represent the mean ± SEM of 3 individual experiments, each in triplicate (n = 8 baboons per group). *P < .05, using an unpaired t test with Welch correction.
Discussion
We analyzed the expression levels of circulating miRNAs in the serum of baboons with experimental endometriosis after treatment with either simvastatin or control. As outlined in our previous report,9 simvastatin treatment partially reduced manifestations of endometriosis, including the combined volume of red, orange-red, and white lesions, decreased cell proliferation Proliferating cell nuclear antigen (PCNA expression), as well as decreased the levels of circulating indicators of inflammation (serum neopterin).9 Although the endometriosis in these baboons was not completely eradicated by this short course of simvastatin treatment, significant improvements were found, including reduced disease volume (combined lesion and pelvic adhesion volume). We report here that several circulating miRNAs concurrently displayed changes reflecting the decrease in endometriosis disease burden following simvastatin treatment. Three miRNAs were expressed differentially in baboons treated with simvastatin compared to untreated endometriotic controls, reflecting a normalization of those altered in women with endometriosis.31 In humans, we previously showed that the levels of miR-150-5p and miR-451a were increased, while miR-3613-5p was decreased significantly in the serum of women with endometriosis. Here, baboons with simvastatin-treated endometriosis displayed the reverse after treatment: lower expression of miR-150-5p and miR-451a, and higher expression of miR-3613-5p compared to the untreated group (ie, levels returning toward normal levels). These parallel patterns support the relevance of circulating miR-150-5p, miR-451a, and miR-3613-3p in endometriosis.
The primate model is a robust proxy for studying endometriosis affecting women. The model facilitates a much better degree of experimental control, granting the ability to control the onset of endometriosis, eliminate confounding variables (comorbidities, medication, etc), and laparoscopically measure lesions more frequently than is possible in human patients. Baboon studies have been instrumental to designing cause and effect experiments that have helped elucidate the mechanisms of endometriosis and how it contributes to subfertility.12,35 This primate model is considered the best preclinical model system in which to test the efficacy of endometriosis therapies and has recently been used in studies of drugs such as aromatase inhibitors36 and inhibitors of inflammatory pathways.37
Mounting evidence links miRNAs to the pathogenesis of endometriosis, with studies reporting regulation of abnormal cell differentiation, invasion, and inflammation.32,38–41 There is a growing collection of miRNAs involved in endometriosis,31,33,41–43 which can regulate mRNA expression in various pathways contributing to pathophysiology of the disease, such as inflammation, neovascularization, invasion, and changes in steroidogenesis. Multiple groups have observed dysregulated expression levels of miRNAs in tissue and blood samples from women with the disease,27,31,33,44 and our study is the first to show altered miRNA expression following endometriosis treatment. In addition to local effects of miRNA in endometrisois, circulating miRNAs may have a hormone-like effect, signaling remote tissues. Endometriosis-derived miRNAs affect macrophages where miR-125b regulates cytokine production that contributes to inflammation associated with this condition.45 Several other miRNAs characterized here have known functions in remote tissues; these functions may be affected by altered delivery of circulating miRNAs. MiR-150 functions in hematopoiesis,46 miR-3613 alters cell proliferation and regulates the cell cycle,47 miR-143 and 145 have a known role in epithelial cell repair and wound healing,48 miR-500a in cell migration and invasion,49 miR-342 in suppressing cell proliferation, migration and invasion,50 and miR-451 in drug resistance.51 The systemic manifestations of endometriosis may be, at least in part, mediated by changes in circulating miRNAs. The systemic effects of these miRNAs may be altered by the changes in circulating level in response to endometriosis treatment. Therapy may relieve some endometriosis symptoms not only by affecting the lesions but also by improving the remote effects mediated by circulating miRNAs.
Other research groups have also observed parallel changes in miRNAs between baboons and humans. Increased expression of miR-29c was measured in eutopic endometrium samples of baboons and women with endometriosis, with in vitro studies suggesting that miR-29c upregulation contributes to progesterone resistance by decreasing levels of FKBP4.52 Nothnick et al performed a comprehensive investigation of miR-451a, showing that circulating miR-451a in serum was upregulated in baboons and humans with endometriosis, and detecting a significant positive correlation between miR-451a levels in serum and in endometriotic lesions.34 This group also measured miR-451a expression in baboon serum over time, with the highest expression, an approximate 20-fold increase, observed at 6 months postinduction of the disease.34 Here, we found that levels of miR-451a in untreated baboon serum at 3 months postinduction were approximately 10-fold higher than in simvastatin-treated baboons. It is encouraging that our study found the same trend of higher expression of miR-451a in the untreated baboons with greater total lesion volume and a concomitant decrease with decreased lesion volume in treated baboons. Differences in study design and sampling time after disease induction likely contribute to the slight variation between miR-451a levels in our primate experiment and the above study. Our previous human study showed that miR-451a expression was increased in patients with endometriosis, with an AUC value of 0.835 to predict presence of the disease.31 A similar AUC value of 0.856 to distinguish women with endometriosis was reported for miR-451a by Nothnick et al.34
As statin are thought to exert their effects on endometriosis by reducing cell invasiveness, neoangiogeneisis, and cell adhesion, the miRNAs that changed in our analysis may be those linked to the pathways altered by this particular drug. In murine studies, miR-150 has been shown to have proangiogenic effects.53 It has also been implicated in cervical cancer proliferation and invasion54 and reported to act as a tumor suppressor.55 MiR-451a has also been shown to act as a tumor suppressor, inhibiting cell proliferation in breast cancer,56 retarding cell migration and invasion in melanoma,57 and inhibiting cell growth and invasion of glioma cells by downregulating the PI3K/AKT activation.58 Circulating miR-451a in humans has been proposed as a potential biomarker for the diagnosis of papillary thyroid carcinomas,59 gastric,60 and breast cancers.61 Other endometriosis treatments act by diverse mechanisms and thus will impact other miRNAs associated with those target pathways. For example, aromatase inhibitors inhibit estrogen synthesis, leading to a decrease in endometriosis. By treating human endometriotic cells in vitro with an aromatase inhibitor, Cho et al found increased expression of let-7f, which led to reduced migration of endometrial cells.62 Similarly, let-7b and miR-125 regulate cytokine production from macrophages in endometriosis.59 The interplay between altered miRNA expression, the posttranscriptional regulation of mRNA targets, and the mechanisms of action of specific therapeutics is an exciting area of inquiry that may lead to identification of new targets to treat endometriosis. Results reported here show that simvastatin treatment of endometriosis in baboons significantly affected expression of miRNAs such as miR-150-5p, miR-451a, and miR-3613-5p. As simvastatin is not a standard treatment for endometriosis,60 future studies will evaluate the changes in miRNA biomarkers in response to typical hormone-based endometriosis treatments such as oral contraceptives or GnRH agonists/antagonists.63,64 As our understanding of the underlying biology of endometriosis and alternate therapies improves, miRNAs may serve not only as biomarkers that can diagnose endometriosis but can also as prognosis markers that can track how the disease responds to different therapies.
To improve patient care, it is critical to be able to monitor the behavior of the disease during the course of treatment. Clinical metrics such as decreasing pelvic pain, dysmenorrhea, and dyspareunia aid in assessing therapeutic efficacy, but having objective measurements of disease-specific biomarkers will indicate how well a given therapy works to eliminate endometriosis. This is the first time that circulating miRNAs have been reported to change in response to endometriosis treatment. Congruence between the baboon model and the human disease implies that these miRNA expression patterns may one day be used to help monitor treatment response in clinical practice. To track and predict therapeutic response in cancer, there are ongoing efforts to develop cancer biomarkers for clinical use in treatment monitoring and detecting recurrence.65–67 Identifying the miRNAs tied to endometriosis may one day reveal predictive biomarkers that can inform how well patients will respond to specific classes of therapeutics, guiding physicians in choosing the most appropriate medications. In conclusion, differential regulation of serum miRNA expression in response to simvastatin treatment against endometriosis implicates prognostic value and response to therapy. Future studies in primates and humans will help clarify which miRNA biomarkers best correlate with therapeutic response, to optimize their clinical utility for managing the disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH U54 HD052668 and R01 HD076422.
ORCID iD: Ramanaiah Mamillapalli, PhD  https://orcid.org/0000-0002-4022-8910
https://orcid.org/0000-0002-4022-8910
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 3. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 4. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Figueira PG, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223–236. [DOI] [PubMed] [Google Scholar]
- 8. Taylor HS, Osteen KG, Bruner-Tran KL. et al. Novel therapies targeting endometriosis. Reprod Sci. 2011;18(9):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor HS, Alderman M, D’Hooghe TM, Fazleabas AT, Antoni DJ. Effect of Simvastatin on Baboon endometriosis. Biol Reprod. 2017;97(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sokalska A, Cress A, Bruner-Tran KL. et al. Simvastatin decreases invasiveness of human endometrial stromal cells. Biol Reprod. 2012;87(1):2, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cakmak H, Basar M, Seval-Celik Y. et al. Statins inhibit monocyte chemotactic protein 1 expression in endometriosis. Reprod Sci. 2012;19(6):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Hooghe TM, Kyama CM, Chai D. et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–161. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Q, Duan J, Olson M, Fazleabas A, Guo SW. Cellular changes consistent with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the progression of experimental endometriosis in baboons. Reprod Sci. 2016;23(10):1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril. 1997;68(4):613–625. [DOI] [PubMed] [Google Scholar]
- 15. Sokalska A, Wong DH, Cress A. et al. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab. 2010;95(7):3453–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ. et al. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod. 2006;75(1):107–111. [DOI] [PubMed] [Google Scholar]
- 17. Sokalska A, Anderson M, Villanueva J. et al. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98(3):E463–E471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril. 2009;92(6):2097–2099. [DOI] [PubMed] [Google Scholar]
- 19. Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril. 2010;94(5):1639–1646.e1631. [DOI] [PubMed] [Google Scholar]
- 20. Yilmaz B, Ozat M, Kilic S. et al. Atorvastatin causes regression of endometriotic implants in a rat model. Reprod Biomed Online. 2010;20(2):291–299. [DOI] [PubMed] [Google Scholar]
- 21. Oktem M, Esinler I, Eroglu D, Haberal N, Bayraktar N, Zeyneloglu HB. High-dose atorvastatin causes regression of endometriotic implants: a rat model. Hum Reprod. 2007;22(5):1474–1480. [DOI] [PubMed] [Google Scholar]
- 22. Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94(7):2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Almassinokiani F, Mehdizadeh A, Sariri E. et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo SS, Ishibashi O, Ishikawa G. et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81(4):717–729. [DOI] [PubMed] [Google Scholar]
- 25. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 26. Pang J, Xiong H, Yang H. et al. Circulating miR-34a levels correlate with age-related hearing loss in mice and humans. Exp Gerontol. 2016;76:58–67. [DOI] [PubMed] [Google Scholar]
- 27. Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103(5):1252–1260 e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112(1):55–59. [DOI] [PubMed] [Google Scholar]
- 29. Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89(6):1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402–409. [DOI] [PubMed] [Google Scholar]
- 32. Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab. 2011;96(12):E1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab. 2013;98(1):281–289. [DOI] [PubMed] [Google Scholar]
- 34. Nothnick WB, Falcone T, Joshi N, Fazleabas AT, Graham A. Serum miR-451a levels are significantly elevated in women with endometriosis and recapitulated in baboons (Papio anubis) with experimentally-induced disease. Reprod Sci. 2017;24(8):1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15(10):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langoi D, Pavone ME, Gurates B, Chai D, Fazleabas A, Bulun SE. Aromatase inhibitor treatment limits progression of peritoneal endometriosis in baboons. Fertil Steril. 2013;99(3):656–662.e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hussein M, Chai DC, Kyama CM. et al. c-Jun NH2-terminal kinase inhibitor bentamapimod reduces induced endometriosis in baboons: an assessor-blind placebo-controlled randomized study. Fertil Steril. 2016;105(3):815–824.e815. [DOI] [PubMed] [Google Scholar]
- 38. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell PS, Parkin RK, Kroh EM. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nothnick WB, Falcone T, Joshi N, Fazleabas AT, Graham A. Serum miR-451a levels are significantly elevated in women with endometriosis and recapitulated in baboons (Papio anubis) with experimentally-induced disease. Reprod Sci. 2016;24(8):1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grechukhina O, Petracco R, Popkhadze S. et al. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4(3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saare M, Rekker K, Laisk-Podar T. et al. Challenges in endometriosis miRNA studies—From tissue heterogeneity to disease specific miRNAs. Biochim Biophys Acta. 2017;1863(9):2282–2292. [DOI] [PubMed] [Google Scholar]
- 43. Nothnick WB. MicroRNAs and endometriosis: distinguishing drivers from passengers in disease pathogenesis. Semin Reprod Med. 2017;35(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod. 2013;28(2):322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS. Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b-5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab. 2018;103(1):64–74. [DOI] [PubMed] [Google Scholar]
- 46. He Y, Jiang X, Chen J. The role of miR-150 in normal and malignant hematopoiesis. Oncogene. 2014;33(30):3887–3893. [DOI] [PubMed] [Google Scholar]
- 47. Zhang D, Liu E, Kang J, Yang X, Liu H. MiR-3613-3p affects cell proliferation and cell cycle in hepatocellular carcinoma. Oncotarget. 2017;8(54):93014–93028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chivukula RR, Shi G, Acharya A. et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157(5):1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo Y, Chen L, Sun C, Yu C. MicroRNA-500a promotes migration and invasion in hepatocellular carcinoma by activating the Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2017;91:13–20. [DOI] [PubMed] [Google Scholar]
- 50. Xue X, Fei X, Hou W, Zhang Y, Liu L, Hu R. miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett. 2018;412:170–178. [DOI] [PubMed] [Google Scholar]
- 51. Li L, Gao R, Yu Y. et al. Tumor suppressor activity of miR-451: identification of CARF as a new target. Sci Rep. 2018;8(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joshi NR, Miyadahira EH, Afshar Y. et al. Progesterone resistance in endometriosis is modulated by the altered expression of microRNA-29c and FKBP4. J Clin Endocrinol Metab. 2016;102(1):jc.2016–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Desjarlais M, Dussault S, Dhahri W, Mathieu R, Rivard A. MicroRNA-150 modulates ischemia-induced neovascularization in atherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2017;37(5):900–908. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Z, Wang J, Li J, Wang X, Song W. MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4. Biomed Pharmacother. 2017;97:511–517. [DOI] [PubMed] [Google Scholar]
- 55. Wang F, Ren X, Zhang X. Role of microRNA-150 in solid tumors. Oncol Lett. 2015;10(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu Z, Miao T, Feng T. et al. miR-451a inhibited cell proliferation and enhanced tamoxifen sensitive in breast cancer via macrophage migration inhibitory factor. Biomed Res Int. 2015;2015:207684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Babapoor S, Fleming E, Wu R, Dadras SS. A novel miR-451a isomiR, associated with amelanotypic phenotype, acts as a tumor suppressor in melanoma by retarding cell migration and invasion. PLoS One. 2014;9(9):e107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nan Y, Han L, Zhang A. et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. [DOI] [PubMed] [Google Scholar]
- 59. Li M, Song Q, Li H, Lou Y, Wang L. Circulating miR-25-3p and miR-451a may be potential biomarkers for the diagnosis of papillary thyroid carcinoma. PLoS One. 2015;10(7):e0132403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Konishi H, Ichikawa D, Komatsu S. et al. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer. 2012;106(4):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luo J, Zhao Q, Zhang W. et al. A novel panel of microRNAs provides a sensitive and specific tool for the diagnosis of breast cancer. Mol Med Rep. 2014;10(2):785–791. [DOI] [PubMed] [Google Scholar]
- 62. Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f–induced cell migration in endometrial cells from women with endometriosis. Fertil Steril. 2016;106(3):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taylor HS, Giudice LC, Lessey BA. et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40. [DOI] [PubMed] [Google Scholar]
- 64. Schrager S, Falleroni J, Edgoose J. Evaluation and treatment of endometriosis. Am Fam Physician. 2013;87(2):107–113. [PubMed] [Google Scholar]
- 65. Hamam R, Hamam D, Alsaleh KA. et al. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8(9):e3045–e3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D. Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: a pilot study. PLoS One. 2015;10(10):e0141279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chong GO, Jeon HS, Han HS. et al. Differential microRNA expression profiles in primary and recurrent epithelial ovarian cancer. Anticancer Res. 2015;35(5):2611–2617. [PubMed] [Google Scholar]