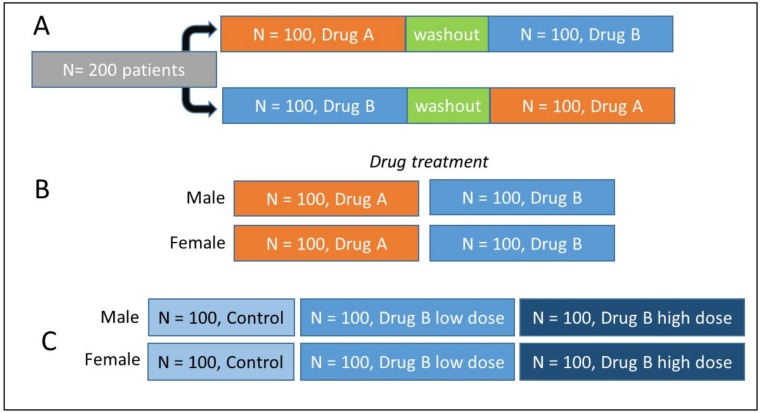
Figure 1.
Common experimental designs. (A) Cross-over design involving a large patient cohort. Two drugs are administered sequentially to each patient, with a crucial washout period between each drug to enable the effects of each drug to be elucidated. (B) Factorial design, where both the gender of the subject and effect of the drug are being studied. (C) Common cross-sectional design in metabolomics studies, comparing controls and two drug dose levels in both genders.