Abstract
The aim of the study was to assess the reproducibility of a short-form, multicomponent dietary questionnaire (SF-FFQ4PolishChildren) in Polish children and adolescents. The study involved 437 children (6–10 years old) and 630 adolescents (11–15 years old) from rural and urban areas of Poland. The self-administered questionnaire was related to nutrition knowledge, dietary habits, active/sedentary lifestyle, self-reported weight and height, and socioeconomic data. The questionnaire was completed with a two-week interval—twice by parents for their children (test and retest for children), twice by adolescents themselves (adolescent’s test and retest) and once by adolescents’ parents (parent’s test). The strength of agreement measured using the kappa statistic was interpreted as follows: 0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, and 0.81–1.00 excellent. Regarding the frequency of consumption of food items and meals, kappa statistics were 0.46–0.81 (the lowest: fruit/mixed fruit and vegetable juices; the highest: Energy drinks) in test–retest for children, 0.30–0.54 (fruit/mixed fruit and vegetable juices; breakfast, respectively) in adolescent’s test–retest, 0.27–0.56 (the lowest: Sweets, fruit, dairy products; the highest: Breakfast) in adolescent’s test and parent’s test. Lower kappa statistics were found for more frequently consumed foods (juices, fruit, vegetables), higher kappa statistics were found for rarely consumed foods (energy drinks, fast food). Across study groups, kappa statistics for diet quality scores were 0.31–0.55 (pro-healthy diet index, pHDI) and 0.26–0.45 (non-healthy diet index, nHDI), for active/sedentary lifestyle items they were 0.31–0.72, for components of the Family Affluence Scale (FAS) they were 0.55–0.93, for BMI categories (based on self-reported weight and height) they were 0.64–0.67, for the nutrition knowledge (NK) of adolescents the kappa was 0.36, for the nutrition knowledge of children’s parents it was 0.62. The Spearman’s correlations for diet quality scores were 0.52–0.76 (pHDI) and 0.53–0.83 (nHDI), for screen time score they were 0.45–0.78, for physical activity score they were 0.51–0.77, for the FAS score they were 0.90–0.93, and for the NK score they were 0.68–0.80. The questionnaire can be recommended to evaluate dietary and lifestyle behaviors among children and adolescents.
Keywords: food frequency questionnaire, reproducibility, reliability, eating behaviors, meal consumption, nutrition knowledge, physical activity, schoolchildren
1. Introduction
Dietary and lifestyle behavior evaluation in children and adolescents is crucial to develop and implement effective policies preventing dietary-related diseases and ensuring public health in the future. Lifestyle habits affect health and are shaped at an early age and track throughout adolescence into adulthood [1,2,3].
Dietary assessment in children and adolescents can be more difficult than in adults [4]. In children, low cognitive abilities, e.g., limited knowledge of food, memory, conceptualization of frequency, may lead to substantial misreporting and, therefore, dietary reporting is conducted by proxies, typically parents or caregivers [4,5,6]. Adolescents are characterized by a high day-to-day variability in diet [5]. They may also perceive long questionnaires as boring or tiring and be less interested in giving valid answers [6]. Short tools allowing quick, easy, low-cost, and reliable assessment of dietary and lifestyle behaviors can be more useful in large epidemiological research and more suitable for children and adolescents than long questionnaires [4,7,8,9]. Shorter questionnaires are described as potentially more advantageous if they can accurately discriminate people with low and high food consumption and identifying groups at risk [10].
Each newly developed or adapted tool should be tested for reproducibility and validity in the target population [10,11,12]. A range of measurement errors affected the reproducibility (random errors) and validity (systematic errors) of dietary methods. Since it is impossible to eliminate them completely, it is important to identify possible sources and to assess the level of errors [12]. For example, one of the common errors in dietary assessment methods is misreporting of foods and/or portion sizes consumed over a specified period of time, leading to underestimation or overestimation of energy and nutrient intake [4,7,12,13].
To the best of our knowledge, there are only a few food frequency questionnaires (FFQs) in Poland for which reproducibility or relative validity among children or adolescents is assessed [14,15,16]. The relative validity of a semi-quantitative FFQ against repeated 24-h dietary recalls was evaluated in Polish children aged 3 years old, showing overestimation of energy and nutrient intake by the FFQ (e.g., median of differences in energy intake between the two methods was 255.4 kcal, and in the range of 7.0–31.0 g for intake of macronutrients) [14]. A non-quantitative FFQ (KomPAN®) was tested in Polish adolescents and adults aged 15–65 years, showing moderate to very good reproducibility of the questionnaire (e.g., kappa statistic for food items was in the range of 0.62–0.84 for the interviewer-administered questionnaire and 0.5–0.78 for the self-administered questionnaire in healthy subjects) [15]. Another non-quantitative FFQ (62-item FFQ-6) was tested in Polish females aged 13–21 years, demonstrating good or very good reproducibility for most food items and acceptable-to-good reproducibility of identification of dietary patterns (e.g., the Spearman correlations were more than 0.50 for 57 out of 62 food items, and for dietary pattern scores were in the range of 0.48–0.84 in the total sample) [16]. There is a lack of tested questionnaires developed for children and younger adolescents. Therefore, the aim of this study was to assess the reproducibility of a short-form, multicomponent dietary questionnaire to assess food frequency consumption, nutrition knowledge, and lifestyle (SF-FFQ4PolishChildren) in Polish schoolchildren aged 6–15 years.
2. Materials and Methods
2.1. Ethical Approval
The study was approved by the Bioethics Committee of the Faculty of Medical Sciences, University of Warmia and Mazury in Olsztyn in 17 June 2010 (resolution no. 20/2010). Informed consent was obtained from parents or legal guardians of schoolchildren.
2.2. Study Design
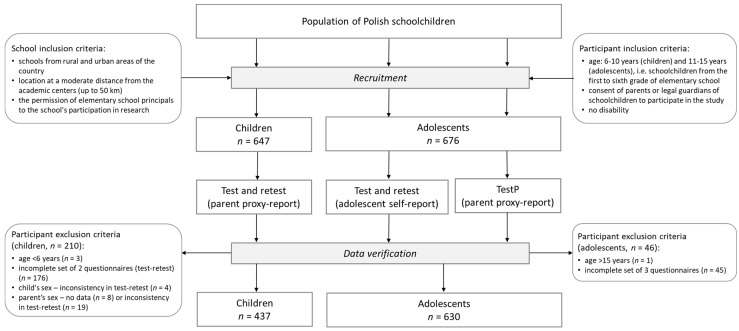
The study was conducted in Poland in autumn 2016. The recruitment was carried out in elementary schools (not randomly selected) located across the whole country, covering rural and urban areas (Figure S1). Inclusion criteria for schools were location at a moderate distance from the academic centers (up to 50 km) and the permission of school principals for the school’s participation in research (Figure 1). Schoolchildren from the first to sixth grade of elementary schools were invited to take part in the study. The self-administered questionnaire was administered in a paper format. The questionnaire was completed with a two-week interval—twice by parents for their children (test–retest for children), twice by adolescents themselves (adolescent’s test–retest), and once by the adolescents’ parents (testP) (Figure 1). In line with previous studies [17,18,19,20,21], the two-week interval was chosen as long enough to avoid recalling previous responses, but short enough to avoid real changes in dietary behaviors, lifestyle, or nutrition knowledge in schoolchildren. Dietary behaviors of schoolchildren, especially adolescents, can change more rapidly than adults [4,5,6].
Figure 1.
Study design and data collection.
2.3. Participants
Initially, 1323 subjects were recruited as a convenience sample, and 256 respondents (19.3% of the sample) were excluded due to: respondent’s age—under 6 years (n = 3) or over 15 years (n = 1), incomplete sets of questionnaires (i.e., less than two for children or less than three for adolescents) (n = 221), child’s sex—inconsistency in test–retest (n = 4), parent’s sex—no data (n = 8) or inconsistency in test–retest (n = 19) (Figure 1). Finally, in total 1067 respondents were included in the study—437 children (6–10 years old) and 630 adolescents (11–15 years old).
2.4. A Short-Form, Multicomponent Dietary Questionnaire (SF-FFQ4PolishChildren)
The SF-FFQ4PolishChildren is a self-administered tool developed for schoolchildren by Kowalkowska, Wadolowska, and Hamulka in 2015 and previously published by Hamulka et al. [22]. The questionnaire consisted of a total of 44 items regarding: Dietary habits (11 items), nutrition knowledge (18 items), active/sedentary lifestyle (3 items), demographics (4 items), the Family Affluence Scale (FAS) components (6 items), anthropometric data (2 items). The reference period of the questionnaire was the previous 12 months.
2.5. Dietary Habits
The habitual frequency of eating two meals (breakfast, a meal at school) and the consumption of nine food items (dairy products, fish, fast food, sweetened soft drinks, fruit/mixed fruit and vegetable juices, energy drinks, vegetables, fruit, sweets) were collected. Respondents reported their breakfast consumption by choosing one of four categories: Less than once a week, 1–3 times/week, 4–6 times/week, every day. A meal at school was considered as the second eating episode of the school day (e.g., lunch, second breakfast). Respondents reported its consumption choosing one of four categories: Less than once a week, 1–2 times/week, 3–4 times/week, every school day (5 times/week). For food items, respondents could choose one of seven consumption frequency categories (converted into daily frequency, times/day): Never/almost never (0), less than once a week (0.06), once a week (0.14), 2–4 times/week (0.43), 5–6 times/week (0.79), every day (1), a few times a day (2) [22].
To evaluate overall diet quality, a pro-healthy diet index (pHDI) and a non-healthy diet index (nHDI) were established based on previous knowledge and other studies [15,23]. The diet quality scores were created a priori by summing the consumption frequencies (times/day) of the following food items: The pHDI—dairy products, fish, vegetables, fruit; the nHDI—fast food, sweetened soft drinks, energy drinks and sweets [22]. Each diet quality score was expressed in % points and categorized as follows: Low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points).
2.6. Nutrition Knowledge
Nutrition knowledge (NK) was assessed based on 18 questions with five response categories, including “I don’t know” [22]. The questions were developed based on a questionnaire described by Whati et al. [24] and adapted to Polish conditions and education [25]. The NK score was calculated for each respondent by summing the points obtained from correct answers (each for 1 point). Respondents were classified into three NK levels: Low (0–5 points), moderate (6–12 points), high (13–18 points). In the children’s group, NK was only assessed for their parents.
2.7. Active/Sedentary Lifestyle
Three questions related to screen time (ST) and physical activity (PA) at school and at leisure time were applied, which had been previously developed for 15–65-year-olds (the KomPAN® questionnaire) [15,23] and adopted for younger respondents. Respondents reported ST, choosing one of six categories (hours/day): <2, 2 to <4, 4 to <6, 6 to <8, 8 to <10, and ≥10. Scores from 0 points for the shortest time (<2 h/day) to 5 points for the longest ST (≥10 h/day) were assigned [22]. For PA at school and PA at leisure time, respondents could choose one of three categories for each type of PA: Low, moderate, high. Based on both types of PA, the total PA level was evaluated by assigning scores from 0 to 5 points (Table 1). Respondents were classified into three categories of the PA level: Low (0–1 points), moderate (2–4 points), and high (5 points).
Table 1.
Categorizing and scoring of the total physical activity level [22].
| Physical Activity at School | Physical Activity at Leisure Time | ||
|---|---|---|---|
| Low | Moderate | High | |
| Low | Low (0 points) | Low (1 points) | Moderate (2 points) |
| Moderate | Low (1 points) | Moderate (3 points) | Moderate (4 points) |
| High | Moderate (2 points) | Moderate (4 points) | High (5 points) |
2.8. The Family Affluence Scale Components
The socioeconomic assessment was based on six questions of FAS (version III) described by the Polish team of the Health Behavior of School-Aged Children (HBSC) international study [26] (Table 2). The FAS score was calculated by summing points assigned for the categories selected by a respondent in each of the questions and ranged from 0 to 9 points [26].
Table 2.
Components of the Family Affluence Scale [26].
| Question | Response Categories | |
|---|---|---|
| 1. | How many computers, laptops, or tablets does your family own? | none (0 points); one (1 point); two (2 points); more than two (2 points) |
| 2. | Does your family own a car, van, or truck? | no (0 points); yes, one (1 point); yes, two or more (2 points) |
| 3. | Does your family have a dishwasher? | no (0 points); yes (1 point) |
| 4. | Do you have your own bedroom? | no (0 points); yes (1 point) |
| 5. | How many bathrooms (room with a bath or shower) are in your home? | none (0 points); one (1 point); two (2 points); more than two (2 points) |
| 6. | Does your home have an outdoor space attached (e.g., garden)? | no (0 points); yes (1 point) |
2.9. Anthropometric Data
Data on self-reported height and body weight were collected, and the body mass index (BMI) was calculated. According to age-sex-specific international standards for children and adolescents [27], respondents were classified into three BMI-for-age categories: Thinness, normal weight, and overweight.
2.10. Statistical Analysis
Means with 95% confidence interval (CI) and percentage distribution of participant characteristics were calculated. Normality of the distribution of continuous variables in the total sample, boys and girls, as well as rural and urban residents was checked by the Kolmogorov–Smirnov test. Urban residents were respondents who indicated one of the following categories of place of residence: “town” or “city (≥100,000 inhabitants)”. The test–retest reproducibility of the questionnaire was assessed as follows: (i) cross-classification analysis and the kappa statistic were calculated for 25 variables: NK level, dietary habits (13 variables), lifestyle (4 variables), FAS components (six variables), BMI categories; (ii) the Spearman’s rank correlation coefficient was calculated for six variables (all in points) in the total sample as well as by sex and place of residence: NK score, pHDI, nHDI, ST score, PA score, FAS; (iii) means were calculated for the same six variables (all in points) in the total sample and by sex and place of residence and then compared between test and retest using Wilcoxon signed-rank test (for two dependent samples); (iv) the Bland–Altman method was used for diet quality scores (pHDI, nHDI) to assess an agreement between test and retest (or parent’s test) in the total sample and by sex groups [28]. Mean difference between test and retest, 95% limits of agreement (LOA), and the Bland–Altman index (percentage of respondents beyond LOA) were calculated. The Bland–Altman index ≤5% indicated good test–retest reproducibility of the diet quality score [28,29]. The strength of correlation was interpreted as follows: 0–0.29 fair, 0.30–0.49 moderate, 0.50–0.69 good, and 0.70–1.00 very good. The strength of agreement measured using the kappa statistic was interpreted as follows: 0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, and 0.81–1.00 excellent [30].
All statistical analyses were performed using STATISTICA software (version 12.0 PL; StatSoft Inc., Tulsa, OK, USA; StatSoft, Cracow, Poland), and p ≤ 0.05 was considered significant.
3. Results
3.1. Participant Characteristics
Participant characteristics in the first administration of the questionnaire are shown in Table 3.
Table 3.
Characteristics of children and adolescents in the first administration of the SF-FFQ4PolishChildren questionnaire.
| Variables | Children Aged 6–10 Years (Questionnaire Filled Out by A Parent) |
Adolescents Aged 11–15 Years (Questionnaire Filled Out by An Adolescent) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Sample size | 437 | 630 | ||
| Sex | ||||
| boys | 211 | 48.3 | 325 | 51.6 |
| girls | 226 | 51.7 | 305 | 48.4 |
| Age (years) 1 | 437 | 8.0 (7.9; 8.1) |
630 | 12.5 (12.4; 12.6) |
| Residence | 437 | 630 | ||
| rural | 231 | 52.9 | 293 | 46.5 |
| urban 2 | 206 | 47.1 | 337 | 53.5 |
| FAS (points) 1 | 431 | 6.2 (6.0; 6.4) |
622 | 6.8 (6.7; 6.9) |
| Nutrition knowledge score (points) 1,3 | 436 | 10.4 (10.1; 10.6) |
626 | 7.2 (6.9; 7.4) |
| Nutrition knowledge level 3 | 436 | 626 | ||
| low | 28 | 6.4 | 191 | 30.5 |
| moderate | 310 | 71.1 | 417 | 66.6 |
| high | 98 | 22.5 | 18 | 2.9 |
| pHDI (%points) 1 | 433 | 31.5 (30.3; 32.8) |
628 | 29.0 (27.9; 30.1) |
| pHDI category | 433 | 628 | ||
| low | 252 | 58.2 | 417 | 66.4 |
| moderate | 175 | 40.4 | 204 | 32.5 |
| high | 6 | 1.4 | 7 | 1.1 |
| nHDI (%points) 1 | 436 | 13.0 (12.2; 13.9) |
627 | 15.1 (14.2; 16.0) |
| nHDI category | 436 | 627 | ||
| low | 416 | 95.4 | 577 | 92.0 |
| moderate | 20 | 4.6 | 49 | 7.8 |
| high | 0 | 0.0 | 1 | 0.2 |
| Screen time score (points) 1 | 437 | 0.4 (0.4; 0.5) |
629 | 0.9 (0.8; 1.0) |
| Screen time category | 437 | 629 | ||
| <2 h/day | 278 | 63.6 | 262 | 41.7 |
| 2 to <4 h/day | 134 | 30.7 | 238 | 37.8 |
| 4 to <6 h/day | 22 | 5.0 | 90 | 14.3 |
| 6 to <8 h/day | 0 | 0.0 | 19 | 3.0 |
| 8 to <10 h/day | 2 | 0.5 | 10 | 1.6 |
| ≥10 h/day | 1 | 0.2 | 10 | 1.6 |
| Physical activity score (points) 1 | 437 | 3.2 (3.0; 3.3) |
629 | 3.4 (3.3; 3.5) |
| Physical activity level | 437 | 629 | ||
| low | 73 | 16.7 | 99 | 15.7 |
| moderate | 304 | 69.6 | 378 | 60.1 |
| high | 60 | 13.7 | 152 | 24.2 |
| Physical activity at school | 437 | 629 | ||
| low | 74 | 16.9 | 68 | 10.8 |
| moderate | 284 | 65.0 | 316 | 50.2 |
| high | 79 | 18.1 | 245 | 39.0 |
| Physical activity at leisure time | 437 | 629 | ||
| low | 31 | 7.1 | 81 | 12.9 |
| moderate | 219 | 50.1 | 284 | 45.2 |
| high | 187 | 42.8 | 264 | 42.0 |
| BMI-for-age | 415 | 595 | ||
| thinness | 76 | 18.3 | 81 | 13.6 |
| normal weight | 263 | 63.4 | 425 | 71.4 |
| overweight | 76 | 18.3 | 89 | 15.0 |
1 mean and 95% confidence interval (CI); 2 urban residents—respondents who indicated one of the following categories of place of residence: “town” or “city (≥100,000 inhabitants)”; FAS—the Family Affluence Scale composed of six questions and ranged from 0–9 points [26]; nutrition knowledge score—evaluated based on 18 questions and ranged 0–18 points [22]; nutrition knowledge level—assessed in three categories: Low (0–5 points), moderate (6–12 points), high (13–18 points); 3 in a group of 6–10-year-old children, nutrition knowledge was assessed in their parents; pHDI (% points)—a pro-healthy diet index composed of four questions (dairy products, fish, vegetables, fruit) and ranged from 0–100 points [22]; pHDI category—low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points); nHDI (% points)—a non-healthy diet index composed of four questions (fast food, sweetened soft drinks, energy drinks, sweets) and ranged from 0–100 points [22]; nHDI category—low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points); screen time score—based on a single question with six response categories and ranged from 0–5 points [22]; physical activity score—based on two questions: Physical activity at school and physical activity at leisure time, and ranged from 0–5 points [22]; physical activity level—assessed in three categories: Low (0–1 points), moderate (2–4 points), high (5 points); BMI-for-age—the age-sex-specific body mass index calculated using self-reported height and weight and assessed in three categories [27].
3.2. Nutrition Knowledge
Cross-classification agreement of the NK level was 85.0% for children’s parents and 72.7% for adolescents (Table 4). The kappa values were 0.62 and 0.36, respectively (Table 5). The Spearman’s correlations for NK score were 0.80 and 0.68, respectively (all <0.05) (Table 6). Correlations for NK score and five other variables (i.e., pHDI, nHDI, ST score, PA score, FAS) by sex and place of residence are shown in Table S1, while comparison of mean values of six variables between test and retest (or parent’s test) in the total sample and by sex and place of residence are shown in Table S2.
Table 4.
Agreement of classification in test and retest of the SF-FFQ4PolishChildren questionnaire (%).
| Variables | Cat. 1 | Children Aged 6–10 Years | Adolescents Aged 11–15 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent (Test–Retest) | Adolescent (Test–Retest) | Adolescent (Test) & Parent (TestP) | |||||||||||
| n | Total Agreement | Misclassification | n | Total Agreement | Misclassification | n | Total Agreement | Misclassification | |||||
| ±1 Cat. | ±2 Cat. or More | ±1 Cat. | ±2 Cat. or More | ±1 Cat. | ±2 Cat. or More | ||||||||
| Nutrition knowledge level 2 | 3 | 432 | 85.0 | 14.8 | 0.2 | 615 | 72.7 | 27.3 | 0.0 | 628 | NA | ||
| Dietary habits | |||||||||||||
| Breakfast | 4 | 436 | 88.8 | 7.8 | 3.4 | 629 | 77.3 | 17.8 | 4.9 | 628 | 80.3 | 12.6 | 7.2 |
| Meal at school | 4 | 435 | 95.4 | 3.9 | 0.7 | 629 | 79.8 | 16.2 | 4.0 | 626 | 81.6 | 14.7 | 3.7 |
| Dairy products | 7 | 435 | 68.0 | 24.8 | 7.1 | 630 | 51.3 | 33.8 | 14.9 | 627 | 44.3 | 34.4 | 21.2 |
| Fish | 7 | 436 | 77.1 | 19.3 | 3.7 | 628 | 57.3 | 31.5 | 11.1 | 627 | 50.9 | 37.0 | 12.1 |
| Fast food | 7 | 436 | 80.7 | 16.7 | 2.5 | 628 | 61.8 | 30.9 | 7.3 | 627 | 58.9 | 27.8 | 13.4 |
| Sweetened soft drinks | 7 | 436 | 64.9 | 23.4 | 11.7 | 628 | 50.8 | 26.8 | 22.5 | 628 | 41.1 | 32.0 | 26.9 |
| Fruit or mixed fruit and vegetable juices | 7 | 434 | 59.0 | 26.7 | 14.3 | 629 | 42.9 | 33.7 | 23.4 | 628 | 43.5 | 30.7 | 25.8 |
| Energy drinks | 7 | 437 | 98.4 | 1.4 | 0.2 | 629 | 80.1 | 12.4 | 7.5 | 628 | 83.9 | 8.4 | 7.6 |
| Vegetables | 7 | 437 | 66.6 | 19.9 | 13.5 | 628 | 45.9 | 32.3 | 21.8 | 627 | 46.3 | 26.3 | 27.4 |
| Fruit | 7 | 432 | 66.2 | 22.7 | 11.1 | 629 | 44.5 | 34.0 | 21.5 | 627 | 42.9 | 28.9 | 28.2 |
| Sweets | 7 | 433 | 70.2 | 21.5 | 8.3 | 629 | 49.4 | 36.1 | 14.5 | 628 | 43.8 | 31.1 | 25.2 |
| pHDI | 3 | 430 | 79.5 | 20.5 | 0 | 625 | 75.8 | 23.5 | 0.6 | 622 | 69.0 | 30.5 | 0.5 |
| nHDI | 3 | 432 | 96.8 | 3.2 | 0.0 | 625 | 92.0 | 7.7 | 0.3 | 623 | 92.3 | 7.7 | 0.0 |
| Active/sedentary lifestyle | |||||||||||||
| Screen time | 6 | 434 | 86.9 | 11.5 | 1.6 | 623 | 64.8 | 24.9 | 10.3 | 625 | 60.0 | 29.9 | 10.1 |
| Physical activity level | 3 | 432 | 86.3 | 13.4 | 0.2 | 622 | 74.8 | 24.6 | 0.6 | 626 | 65.3 | 32.1 | 2.6 |
| Physical activity at school | 3 | 435 | 84.4 | 14.5 | 1.1 | 622 | 74.6 | 24.0 | 1.4 | 627 | 64.6 | 29.8 | 5.6 |
| Physical activity at leisure time | 3 | 432 | 83.8 | 15.3 | 0.9 | 623 | 71.3 | 27.0 | 1.8 | 626 | 59.9 | 37.9 | 2.2 |
| FAS components | |||||||||||||
| How many computers, laptops, or tablets does your family own? | 4 | 433 | 87.1 | 11.5 | 1.4 | 626 | 83.7 | 15.2 | 1.1 | 623 | 75.6 | 20.5 | 3.9 |
| Does your family own a car, van, or truck? | 3 | 433 | 91.9 | 7.6 | 0.5 | 627 | 86.3 | 11.5 | 2.2 | 622 | 83.6 | 13.0 | 3.4 |
| Does your family have a dishwasher? | 2 | 435 | 97.5 | 2.5 | 625 | 95.8 | 4.2 | 622 | 95.5 | 4.5 | |||
| Do you have your own bedroom? | 2 | 435 | 96.1 | 3.9 | 622 | 95.8 | 4.2 | 620 | 92.9 | 7.1 | |||
| How many bathrooms (room with a bath or shower) are in your home? | 4 | 434 | 95.2 | 4.1 | 0.7 | 628 | 90.9 | 7.5 | 1.6 | 624 | 89.4 | 9.9 | 0.6 |
| Does your home have an outdoor space attached (e.g., garden)? | 2 | 432 | 95.1 | 4.9 | 625 | 95.2 | 4.8 | 622 | 95.2 | 4.8 | |||
| BMI-for-age | 3 | 407 | 89.4 | 10.6 | 0.0 | 583 | 90.2 | 9.6 | 0.2 | 585 | 92.1 | 7.7 | 0.2 |
1 number of categories in the question; cat.—categories; nutrition knowledge level—based on 18 questions and assessed in three categories: Low (5 points), moderate (12 points), high (13–18 points) [22]; 2 in a group of 6–10-year-old children, nutrition knowledge was assessed in their parents; pHDI—a pro-healthy diet index composed of four questions (dairy products, fish, vegetables, fruit) and assessed in three categories: Low (0–33.32% points), moderate (33.33–66.65% points), high (66.6–100% points) [22]; nHDI—a non-healthy diet index composed of four questions (fast food, sweetened soft drinks, energy drinks, sweets) and assessed in three categories: Low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points) [22]; physical activity level—based on two questions: Physical activity at school and physical activity at leisure time and assessed in three categories: Low (0–1 points), moderate (2–4 points), high (5 points) [22]; FAS components—six questions of the Family Affluence Scale [26]; BMI-for-age—the age-sex-specific body mass index calculated using self-reported height and weight and assessed in three categories [27]; NA—not applied.
Table 5.
Kappa statistics for test and retest of the SF-FFQ4PolishChildren questionnaire.
| Variables | Cat. 1 | Children Aged 6–10 Years |
Adolescents Aged 11–15 Years |
|
|---|---|---|---|---|
| Parent (Test–Retest) |
Adolescent (Test–Retest) |
Adolescent (Test) & Parent (TestP) |
||
| Sample size | 437 | 630 | 628 | |
| Nutrition knowledge level 2 | 3 | 0.62 | 0.36 | NA |
| Dietary habits | ||||
| Breakfast | 4 | 0.70 | 0.54 | 0.56 |
| Meal at school | 4 | 0.78 | 0.53 | 0.46 |
| Dairy products | 7 | 0.54 | 0.37 | 0.27 |
| Fish | 7 | 0.67 | 0.42 | 0.32 |
| Fast food | 7 | 0.68 | 0.43 | 0.33 |
| Sweetened soft drinks | 7 | 0.56 | 0.39 | 0.28 |
| Fruit or mixed fruit and vegetable juices | 7 | 0.46 | 0.30 | 0.30 |
| Energy drinks | 7 | 0.81 | 0.44 | 0.45 |
| Vegetables | 7 | 0.56 | 0.31 | 0.31 |
| Fruit | 7 | 0.53 | 0.31 | 0.27 |
| Sweets | 7 | 0.59 | 0.36 | 0.27 |
| pHDI | 3 | 0.55 | 0.44 | 0.31 |
| nHDI | 3 | 0.45 | 0.35 | 0.26 |
| Active/sedentary lifestyle | ||||
| Screen time | 6 | 0.72 | 0.46 | 0.35 |
| Physical activity level | 3 | 0.68 | 0.52 | 0.36 |
| Physical activity at school | 3 | 0.69 | 0.54 | 0.40 |
| Physical activity at leisure time | 3 | 0.69 | 0.51 | 0.31 |
| FAS components | ||||
| How many computers, laptops, or tablets does your family own? | 4 | 0.79 | 0.69 | 0.55 |
| Does your family own a car, van, or truck? | 3 | 0.84 | 0.76 | 0.70 |
| Does your family have a dishwasher? | 2 | 0.93 | 0.89 | 0.87 |
| Do you have your own bedroom? | 2 | 0.90 | 0.83 | 0.74 |
| How many bathrooms (room with a bath or shower) are in your home? | 4 | 0.88 | 0.84 | 0.79 |
| Does your home have an outdoor space attached (e.g., garden)? | 2 | 0.85 | 0.85 | 0.84 |
| BMI-for-age | 3 | 0.67 | 0.64 | 0.65 |
1 Cat.—number of categories in the question; nutrition knowledge level—based on 18 questions and assessed in three categories: Low (0–5 points), moderate (6–12 points), high (13–18 points) [22]; 2 in a group of 6–10-year-old children, nutrition knowledge was assessed in their parents; pHDI—a pro-healthy diet index composed of four questions (dairy products, fish, vegetables, fruit) and assessed in three categories: Low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points) [22]; nHDI—a non-healthy diet index composed of four questions (fast food, sweetened soft drinks, energy drinks, sweets) and assessed in three categories: Low (0–33.32% points), moderate (33.33–66.65% points), high (66.66–100% points) [22]; physical activity level—based on two questions: Physical activity at school and physical activity at leisure time, and assessed in three categories: Low (0–1 points), moderate (2–4 points), high (5 points) [22]; FAS components—six questions of the Family Affluence Scale [26]; BMI-for-age—the age-sex-specific body mass index calculated using self-reported height and weight and assessed in three categories [27]; NA—not applied.
Table 6.
Correlation coefficients (r)1 between test and retest of the SF-FFQ4PolishChildren questionnaire.
| Variables (All in Points) |
Range of Points | Children Aged 6–10 Years (n = 437) |
Adolescents Aged 11–15 Years (n = 630) |
|
|---|---|---|---|---|
| Parent (Test–Retest) |
Adolescent (Test–Retest) |
Adolescent (Test) & Parent (TestP) |
||
| Nutrition knowledge score 2 | 0–18 | 0.80 | 0.68 | NA |
| pHDI | 0–100 | 0.76 | 0.63 | 0.52 |
| nHDI | 0–100 | 0.83 | 0.68 | 0.53 |
| Screen time score | 0–5 | 0.78 | 0.58 | 0.45 |
| Physical activity score | 0–5 | 0.77 | 0.71 | 0.51 |
| FAS | 0–9 | 0.93 | 0.91 | 0.90 |
1r—the Spearman’s rank correlation coefficient (all <0.05); nutrition knowledge score—evaluated based on 18 questions [22]; 2 in a group of 6–10-year-old children, nutrition knowledge was assessed in their parents; pHDI—a pro-healthy diet index composed of four questions (dairy products, fish, vegetables, fruit) [22]; nHDI—a non-healthy diet index composed of four questions (fast food, sweetened soft drinks, energy drinks, sweets) [22]; screen time score—based on a single question with six response categories [22]; physical activity score—based on two questions: Physical activity at school and physical activity at leisure time [22]; FAS—the Family Affluence Scale composed of six questions [26]; NA—not applied.
3.3. Dietary Habits
The proportion of respondents classified into the same frequency category was 59.0–98.4% (the lowest: Fruit/mixed fruit and vegetable juices; the highest: Energy drinks) for children, 42.9–80.1% (fruit/mixed fruit and vegetable juices; energy drinks, respectively) in the adolescent’s test–retest, 41.1–83.9% (sweetened soft drinks; energy drinks, respectively) in the adolescent’s test and parent’s test (Table 4). Kappa was 0.46–0.81 (fruit/mixed fruit and vegetable juices; energy drinks, respectively) in test–retest for children, 0.30–0.54 (fruit/mixed fruit and vegetable juices; breakfast, respectively) in the adolescent’s test–retest, 0.27–0.56 (the lowest: Sweets, fruit, dairy products; the highest: Breakfast) in the adolescent’s test and parent’s test (Table 5).
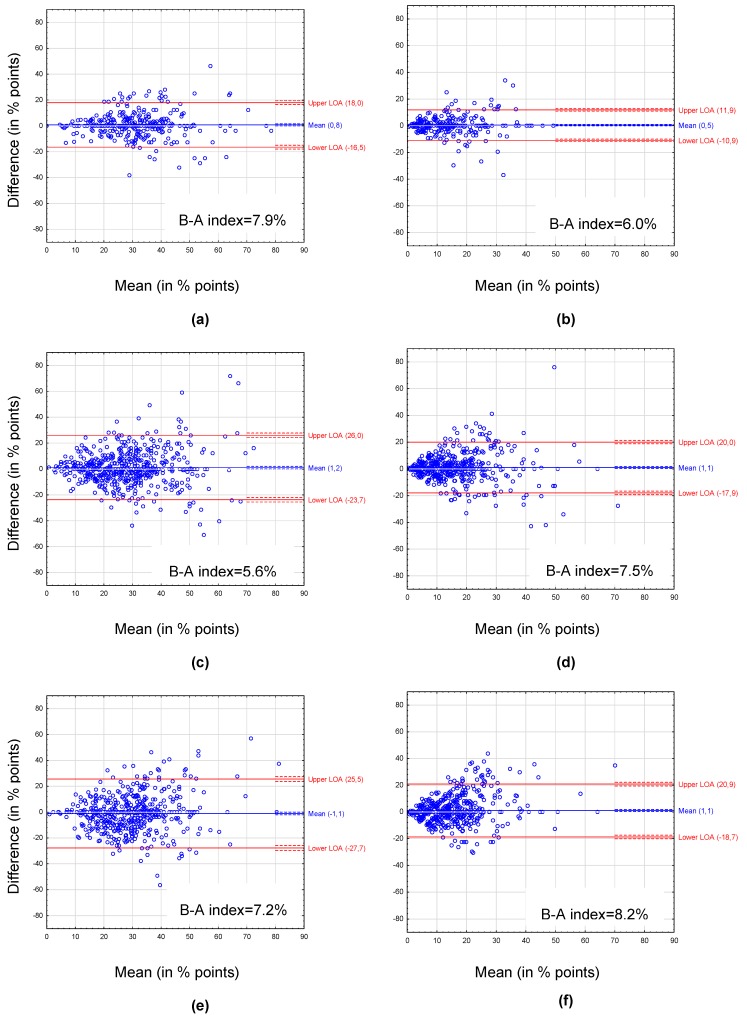
Cross-classification agreement for pHDI was 79.5% for children, 75.8% in adolescent’s test–retest, and 69.0% in the adolescent’s test and parent’s test; for nHDI: 96.8%, 92.0%, and 92.3%, respectively (Table 4). Kappa values were 0.55, 0.44, 0.31 for pHDI and 0.45, 0.35, 0.26 for nHDI, respectively (Table 5). The Spearman’s correlations were 0.76, 0.63, 0.52 for pHDI and 0.83, 0.68, 0.53 for nHDI, respectively (all <0.05) (Table 6). The Bland–Altman plots showed mean difference ranged from −1.1% points for pHDI in the adolescent’s test and parent’s test to 1.2% points for pHDI in the adolescent’s test–retest (Figure 2). The Bland–Altman index ranged from 5.6% for pHDI in the adolescent’s test–retest to 8.2% for nHDI in the adolescent’s test and parent’s test. Results of the Bland–Altman methods by sex groups are shown in supplementary materials (Figures S2 and S3).
Figure 2.
Bland–Altman plots for the pro-healthy diet index (pHDI; left panel) and the non-healthy diet index (nHDI; right panel) between the first and the second administration of the questionnaire: (a) pHDI in children aged 6–10 years (test and retest), (b) nHDI in children aged 6–10 years (test and retest), (c) pHDI in adolescents aged 11–15 years (test and retest), (d) nHDI in adolescents aged 11–15 years (test and retest), (e) pHDI in adolescents aged 11–15 years and their parents (test and testP), (f) nHDI in adolescents aged 11–15 years and their parents (test and testP). Mean—mean difference between the first and the second administration of the questionnaire (blue solid line) with 95% CI (dashed lines). LOA—95% limits of agreement between the first and the second administration of the questionnaire (red solid lines) with 95% CI (dashed lines). B-A index—the Bland–Altman index calculated as percentage of respondents beyond LOA.
3.4. Active/Sedentary Lifestyle
For PA and ST, cross-classification agreement was 83.8–86.9% for children, 64.8–74.8% in the adolescent’s test–retest, 59.9–65.3% in the adolescent’s test and parent’s test (Table 4). Kappa values were 0.68–0.72 for children, 0.46–0.54 in the adolescent’s test–retest, 0.31–0.40 in the adolescent’s test and parent’s test (Table 5). The Spearman’s correlations for ST score were 0.78 for children, 0.58 in the adolescent’s test–retest, 0.45 in the adolescent’s test and parent’s test, while the correlations for PA score were 0.77, 0.71 and 0.51, respectively (all <0.05) (Table 6).
3.5. Socioeconomic Data
Regarding FAS components, cross-classification agreement was 87.1–97.5% for children, 83.7–95.8% in the adolescent’s test–retest and 75.6–95.5% in the adolescent’s test and parent’s test (Table 4). The kappa statistics were 0.79–0.93 for children, 0.69–0.89 in the adolescent’s test–retest, 0.55–0.87 in the adolescent’s test and parent’s test (Table 5). The Spearman’s correlations for the FAS score were 0.93, 0.91 and 0.90, respectively (all <0.05) (Table 6).
3.6. Anthropometric Data
Cross-classification agreement of the BMI categories was 89.4% for children, 90.2% in the adolescent’s test–retest, 92.1% in the adolescent’s test and parent’s test (Table 4). The kappa values were 0.67, 0.64, and 0.65, respectively (Table 5).
4. Discussion
The study showed moderate-to-excellent reproducibility, measured with kappa statistic, for all 25 analyzed items and scores in children (with parents as proxy reporters), 17 of 25 of items/scores (68%) in adolescents and 10 of 24 of items/scores (42%) in the adolescent’s test and parent’s test. In children, the questionnaire demonstrated moderate reproducibility for most foods and diet quality scores, while good-to-excellent for other items/scores related to meal consumption frequency, nutrition knowledge, active/sedentary lifestyle, BMI categories, and FAS components. For adolescents, the reproducibility was fair-to-moderate for all dietary habits, nutrition knowledge, active/sedentary lifestyle, and good-to-excellent for other items. When the adolescent’s test was compared to the parent’s test, the reproducibility was slightly weaker than when the adolescent’s test and retest were compared. This may be explained by the growing independence of adolescents and lower parental knowledge regarding the pupils’ diets due to spending less time together [6].
The test–retest reproducibility for food items found in the present study in children (agreement: 59.0–98.4%, kappa: 0.46–0.81) and adolescents (42.9–80.1%, kappa: 0.30–0.44) was similar or higher than previously reported [31,32,33,34,35,36,37,38]. Parent-administered semi-quantitative FFQs to assess children’s diet demonstrated cross-classification agreement, which ranged from 33–69% in Danish children [32] and 62.1–99.4% in Spanish children [37], but it ranged from 44–82% in New Zealand children using a short non-quantitative FFQ [35]. Lower reproducibility of food consumption frequency was found in children from six European countries (kappa: 0.23–0.68) [39]. For adolescents, the cross-classification agreement found in other reproducibility studies was similar or lower than our findings and ranged from: 29–58% in Italian adolescents [33], 36–55% in Norwegian adolescents [34], 37–87% in Belgian adolescents [36], 45–77% in Danish adolescents [31] and 46–88% in New Zealand adolescents [38], while the kappa values for foods obtained in adolescents were higher compared to our findings: 0.21–0.66 [33], 0.23–0.71 [31], 0.43–0.70 [36].
The present study showed lower reproducibility for more frequently consumed foods (juices, fruit, vegetables) and higher reproducibility for rarely consumed foods (energy drinks, fast food). Similarly, lower test–retest agreement was demonstrated for frequently consumed foods (e.g., white bread, wholemeal bread, fruit juices) and higher agreement for rarely consumed foods (e.g., porridge, fish, energy drinks) in Danish adolescents [31] and Polish adolescents and adults [15]. Estimating the consumption frequency of foods eaten less frequently or never could be easier than those eaten more often and included in various dishes and meals [11,31].
Since the present study showed relatively low reproducibility for certain food items, e.g., fruit and vegetables, the use of diet quality scores (developed based on the questionnaire) seems to be more appropriate than the use of single food items. The advantage of using diet scores, as a comprehensive approach, and their utility to study diet–health relationships were highlighted previously [40,41]. In the present study, the Bland–Altman plots showed very good test–retest reproducibility of diet quality scores at the group level (mean differences from −1.1% to 1.2% points), but moderate reproducibility at the individual level—the 95% LOAs were relatively wide, and values of the Bland–Altman index were slightly higher than 5% [28,29]. Better agreement between test and retest of the questionnaire was observed at low values of diet quality scores than at moderate or high values of pHDI or nHDI. These findings may indicate better test–retest reproducibility of the questionnaire in terms of dietary behaviors in children and adolescents with stable but restrictive eating habits (consuming key foods, healthy or unhealthy, with low frequency and/or consuming only a few selected foods with higher frequency). Better test–retest reproducibility of FFQs for foods consumed rarely or never has been reported in other studies [15,16,31]. The differences between both administrations of the questionnaire were more scattered in adolescents than in children, and the most between the adolescent’s test and parent’s test, which confirms the results of other statistical analyses. Furthermore, most of the mean differences showed an overestimation of diet quality scores in the test compared to the retest. Negative mean differences were found only for the pHDI between the adolescent’s test and the parent’s test in the total sample as well as in sex groups, which may indicate an overestimation of pHDI in parental reporting compared to adolescent self-reporting. A literature review of McPherson et al. [42] showed that the results from the first administration of the questionnaire usually tend to be higher compared to subsequent administrations. In turn, adolescents are characterized by less structured food habits that can change rapidly, eating more out-of-home and growing independence from parents, which can lead to less knowledge of parents about their children’s diet [4,6]. A parental misperception of the child’s diet quality—an overestimation of “healthy” food choices and/or underestimation of less “healthy” food consumption—has been noted in other research [11,43]. All this together can explain the greater discrepancies between test and retest in adolescents, as well as between adolescents and their parents, than in both administrations of the questionnaire in children. Compared to our findings obtained by various statistics (pHDI: Agreement: 69.0–79.5%, kappa: 0.31–0.55, r = 0.52–0.76; nHDI: Agreement: 92.0–96.8%, kappa: 0.26–0.45, r = 0.53–0.83), other studies demonstrated similar test–retest reproducibility for FFQ-based diet scores in: Flemish children (kappa: 0.61; r = 0.88) [44], New Zealand adolescents (agreement: 60%) [17], Norwegian adolescents (agreement: 87.6%; kappa: 0.465) [40] and Norwegian parents of toddlers (kappa: 0.52; r = 0.80) [18].
For NK levels, good reproducibility in children’s parents and fair reproducibility in adolescents was found. For NK score, the strength of the Spearman’s correlation between test and retest was very good in children’s parents and good in adolescents. Means of NK score were different in adolescent’s test–retest only. It may be speculated that the first administration of the questionnaire created interest in this knowledge, causing an increase in the adolescents’ NK in the second administration. Relatively weak reproducibility for single NK items was found in Australian schoolchildren (intra-class correlation (ICC): 0.16–0.36) [45], U.S. adolescents (kappa: 0.30–0.56) [19]. High reproducibility of NK was demonstrated in Belgian schoolchildren (NK score: ICC = 0.76) [20], Italian children and adolescents (Pearson’s correlation coefficient (r): 0.87) [18] or adolescents (r = 0.80) [46].
Active/sedentary lifestyle items showed high reproducibility, especially in children. In adolescents, the agreement was moderate, but fair when the adolescent’s test was compared to parental reporting. For ST score, the strength of the Spearman’s correlation between test and retest was very good in children and good in adolescents, but moderate when the adolescent’s test was compared to parental reporting. For PA score, the strength of correlation was very good in both children and adolescents and good between the adolescent’s test and parent’s test. Similar results of correlations between both administrations of the questionnaire were observed in sex groups as well as in rural and urban residents. High test–retest reproducibility was demonstrated for PA in U.S. adolescents (agreement: 66–89%) [19], PA and lifestyle in Italian children and adolescents (r = 0.70) [21], or adolescents (r = 0.88) [46]. Parental awareness of children’s PA was low; most parents overestimated their child’s PA [47]. Regarding sedentary behaviors, among Norwegian schoolchildren test–retest reproducibility was moderate for weekly scores (r = 0.66–0.73): TV/DVD use, computer/electronic game use, and total ST [48] and was slightly lower for single ST items (r = 0.50–0.65) [49].
For FAS components and the FAS score, good-to-excellent reproducibility was found across study groups, except for the component regarding family computers in the adolescent’s test and parent’s test (kappa: 0.55). Similar results were found in a previous validation study in Polish adolescents and their parents [26]. For FAS components, moderate-to-excellent agreement (kappa: 0.58–0.83) between the adolescents and parents and excellent (0.83–0.95) in the adolescent’s test–retest were found with the lowest reproducibility for family computers [26]. In 11-year-olds and their parents from six European countries, validation of FAS II (with the same three components as in FAS III) showed high reproducibility of FAS components with relatively low agreement for family computers (kappa for six countries: 0.68, Poland: 0.48) [50].
For BMI categories calculated from self-reported data, high cross-classification agreement (89.4–92.1%) and good inter-rater reliability (kappa: 0.64–0.67) were shown. The reproducibility was very similar across study age groups and between the adolescent’s test and parent’s test. Given the overall importance of body image concern, dieting behaviors, peer and media influence in adolescence [6], greater differences between the values reported by adolescents and their parents were expected. Parental reports of weight and height to assess children’s BMI are cost-efficient and often used in large-scale surveys [51,52]. However, comparing our findings with the results of other research is difficult because most of the previous studies referred to self-reported values compared to measured values [51,52,53].
Strengths and Limitations
Several strengths and limitations of this study should be emphasized for future research. The study was conducted using a large sample of over a thousand 6–15-year-old subjects, greater than samples described in other reproducibility studies among schoolchildren [31,33,38,46]. Although this was not a representative national sample, it covered rural and urban areas in all macro-regions of the country [54]. To describe adolescents, the study involved parents and adolescents themselves, which allowed the reproducibility of the data collected in the retest among those groups to be verified and a better approach for adolescents to be selected. Since another strength of the study is assessing the reproducibility of single questions as well as total scores, our findings show more options of the questionnaire application. Moreover, a variety of statistical methods were used to strengthen the conclusions and facilitate comparison of our results with others.
As the study limitation, involving only parents to describe children’s dietary and lifestyle behaviors should be considered. However, this technique of data collection is in line with the recommendations for dietary assessment using questionnaires in children [5,55,56]. Furthermore, only self-reported data were collected with this questionnaire, so misreporting and social desirability biases should be taken into account [12]. Misreporting is one of the most commonly reported measurement errors in dietary assessment methods [4,7,12,13]. Respondents may misreport certain foods systematically—those with low consumption of “healthy” foods may tend to overreport their intake, while those with high consumption of “unhealthy” foods may tend to underreport them [12]. Similarly, possibility of incorrect perception of body image in some respondents and a social desirability bias may not contribute to a lower reproducibility of the classification to BMI category. Such respondents may under-report their body weight and over-report their height to the same extent in both administrations of the questionnaire. Although testing reproducibility of a questionnaire reflects random errors, validation of the tool provides information on systematic errors that are more difficult to control [12]. Therefore, further study to validate the questionnaire against biomarkers and/or other dietary methods as the reference as well as using measured anthropometric data should be conducted.
5. Conclusions
The study showed moderate-to-excellent reproducibility of the questionnaire items and scores with some exceptions. Worse reproducibility was found for more frequently consumed foods, such as juices, fruit, and vegetables. To describe adolescents, the reproducibility was better when the questionnaire was completed by adolescents than parents. The questionnaire can be recommended to evaluate dietary and lifestyle behaviors among children and adolescents.
Acknowledgments
Thanks are expressed to the participants for their contributions to the study and Ewa Piatkowska from the University of Agriculture in Krakow for participation in data collection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/12/2929/s1, Figure S1: The academic centers (including cities and villages around) where data were collected, Table S1: Correlation coefficients (r) between test and retest of the SF-FFQ4PolishChildren questionnaire by sex and place of residence, Figure S2: Boys: Bland–Altman plots for the pro-healthy diet index (pHDI; left panel) and the non-healthy diet index (nHDI; right panel) between the first and the second administration of the questionnaire, Figure S3: Girls: Bland–Altman plots for the pro-healthy diet index (pHDI; left panel) and the non-healthy diet index (nHDI; right panel) between the first and the second administration of the questionnaire, Table S2: Means and 95% confidence interval (CI) in test and retest of the SF-FFQ4PolishChildren questionnaire in the total sample and by sex and place of residence.
Author Contributions
J.K., L.W. and J.H. were responsible for the conceptualization of the study; J.K. and L.W. were responsible for the methodology of the study; J.H., N.W., M.C.-M., W.K., M.B., J.S., S.N., I.D., A.K., E.P.-S., E.C., J.C., M.K., A.D., D.L. and M.J.-B. were responsible for the software; L.W. was responsible for the validation; J.K. was responsible for the formal analysis; J.H., N.W., M.C.-M., W.K., M.B., J.S., S.N., I.D., A.K., E.P.-S., E.C., J.C., M.K., A.D., D.L. and M.J.-B. were responsible for the investigation; J.K. was involved in the resources; J.K., L.W. and J.H. were involved in the data curation; J.K. and L.W. were involved in the interpretation of data; J.K. was responsible for writing—original draft preparation; L.W. and J.H. were involved in writing—review and editing; J.K. was responsible for the data visualization; L.W. and J.H. were responsible for the supervision; L.W. and J.H. were responsible for the project administration; L.W., J.H., M.C.-M., W.K., M.B., J.S., S.N., A.K., E.C., J.C., M.K., A.D. and D.L. were responsible for the funding acquisition. All authors read and approved the final manuscript.
Funding
Project financially supported by Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Craigie A.M., Lake A.A., Kelly S.A., Adamson A.J., Mathers J.C. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas. 2011;70:266–284. doi: 10.1016/j.maturitas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Movassagh E.Z., Baxter-Jones A.D.G.G., Kontulainen S., Whiting S.J., Vatanparast H. Tracking dietary patterns over 20 years from childhood through adolescence into young adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients. 2017;9:990. doi: 10.3390/nu9090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suppli C.H., Due P., Henriksen P.W., Rayce S.L.B., Holstein B.E., Rasmussen M. Low vigorous physical activity at ages 15, 19 and 27: Childhood socio-economic position modifies the tracking pattern. Eur. J. Public Health. 2013;23:19–24. doi: 10.1093/eurpub/cks040. [DOI] [PubMed] [Google Scholar]
- 4.Thompson F., Subar A. Dietary assessment methodology. In: Coulston A.M., Boushey C.J., editors. Nutrition in the Prevention and Treatment of Disease. 2nd ed. Academic Press; San Diego, CA, USA: 2008. pp. 3–39. [Google Scholar]
- 5.Livingstone M.B.E., Robson P.J., Wallace J.M.W. Issues in dietary intake assessment of children and adolescents. Br. J. Nutr. 2004;92:S213–S222. doi: 10.1079/BJN20041169. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Rodrigo C., Escauriaza B.A., Bartrina J.A., Allúe I.P. Dietary assessment in children and adolescents: Issues and recommendations. Nutr. Hosp. 2015;31:76–83. doi: 10.3305/nh.2015.31.sup3.8755. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization (FAO) Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings. FAO; Rome, Italy: 2018. [Google Scholar]
- 8.Golley R.K., Bell L.K., Hendrie G.A., Rangan A.M., Spence A., McNaughton S.A. Validity of short food questionnaire items to measure intake in children and adolescents: A systematic review. J. Hum. Nutr. Diet. 2017;30:36–50. doi: 10.1111/jhn.12399. [DOI] [PubMed] [Google Scholar]
- 9.Lillegaard I.T.L., Øverby N.C., Andersen L.F. Evaluation of a short food frequency questionnaire used among Norwegian children. Food Nutr. Res. 2012;56:6399–6407. doi: 10.3402/fnr.v56i0.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cade J., Thompson R., Burley V., Warm D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002;5:567–587. doi: 10.1079/PHN2001318. [DOI] [PubMed] [Google Scholar]
- 11.Cade J.E., Burley V.J., Warm D.L., Thompson R.L., Margetts B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004;17:5–22. doi: 10.1079/NRR200370. [DOI] [PubMed] [Google Scholar]
- 12.Gibson R. Principles of Nutritional Assessment. 2nd ed. Oxford University Press; New York, NY, USA: 2005. [Google Scholar]
- 13.Tabacchi G., Filippi A.R., Amodio E., Jemni M., Bianco A., Firenze A., Mammina C. A meta-analysis of the validity of FFQ targeted to adolescents. Public Health Nutr. 2016;19:1168–1183. doi: 10.1017/S1368980015002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sochacka-Tatara E., Pac A. Relative validity of a semi-quantitative FFQ in 3-year-old Polish children. Public Health Nutr. 2014;17:1738–1744. doi: 10.1017/S1368980013002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalkowska J., Wadolowska L., Czarnocinska J., Czlapka-Matyasik M., Galinski G., Jezewska-Zychowicz M. Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN) in Polish Adolescents and Adults. Nutrients. 2018;10:1845. doi: 10.3390/nu10121845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedzwiedzka E., Wadolowska L., Kowalkowska J. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13–21-Year-Old Females. Nutrients. 2019;11:2183. doi: 10.3390/nu11092183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong J.E., Parnell W.R., Howe A.S., Black K.E., Skidmore P.M. Development and validation of a food-based diet quality index for New Zealand adolescents. BMC Public Health. 2013;13:562–571. doi: 10.1186/1471-2458-13-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjørnarå H.B., Hillesund E.R., Torstveit M.K., Stea T.H., Øverby N.C., Bere E. An assessment of the test-retest reliability of the New Nordic Diet score. Food Nutr. Res. 2015;59:28397–28404. doi: 10.3402/fnr.v59.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelscher D.M., Day R.S., Kelder S.H., Ward J.L. Reproducibility and validity of the secondary level School-Based Nutrition Monitoring student questionnaire. J. Am. Diet. Assoc. 2003;103:186–194. doi: 10.1053/jada.2003.50031. [DOI] [PubMed] [Google Scholar]
- 20.Vereecken C., De Pauw A., Van Cauwenbergh S., Maes L. Development and test-retest reliability of a nutrition knowledge questionnaire for primary-school children. Public Health Nutr. 2012;15:1630–1638. doi: 10.1017/S1368980012002959. [DOI] [PubMed] [Google Scholar]
- 21.Grosso G., Mistretta A., Turconi G., Cena H., Roggi C., Galvano F. Nutrition knowledge and other determinants of food intake and lifestyle habits in children and young adolescents living in a rural area of Sicily, South Italy. Public Health Nutr. 2013;16:1827–1836. doi: 10.1017/S1368980012003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamulka J., Wadolowska L., Hoffmann M., Kowalkowska J., Gutkowska K. Effect of an education program on nutrition knowledge, attitudes toward nutrition, diet quality, lifestyle, and body composition in polish teenagers. The ABC of healthy eating project: Design, protocol, and methodology. Nutrients. 2018;10:1439. doi: 10.3390/nu10101439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawecki J. In: Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing Nutritional Data. Gawecki J., editor. Committee of Human Nutrition Science, Polish Academy of Sciences; Olsztyn, Poland: 2018. pp. 1–52. [Google Scholar]
- 24.Whati L., Senekal M., Steyn N., Nel J., Lombard C., Norris S. Development of a reliable and valid nutritional knowledge questionnaire for urban South African adolescents. Nutrition. 2005;21:76–85. doi: 10.1016/j.nut.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.National Food and Nutrition Institute (Poland) Pyramid of Healthy Nutrition and Physical Activity. National Food and Nutrition Institute; Warsaw, Poland: 2018. [(accessed on 18 January 2019)]. Available online: http://www.izz.waw.pl/attachments/article/555/Piramida IZZ 1.pdf. [Google Scholar]
- 26.Mazur J. Family Affluence Scale—Validation study and suggested modification. Hygeia Public Health. 2013;48:211–217. [Google Scholar]
- 27.Cole T.J., Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 29.British Standards Institution . Precision of Test Methods 1: Guide for the Determination and Reproducibility for a Research Test Method (BS 597, Part 1) BSI; London, UK: 1975. [Google Scholar]
- 30.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 31.Bjerregaard A.A., Tetens I., Olsen S.F., Halldorsson T.I. Reproducibility of a web-based FFQ for 13- to 15-year-old Danish adolescents. J. Nutr. Sci. 2016;5:1–7. doi: 10.1017/jns.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buch-Andersen T., Perez-Cueto Eulert F.J.A., Toft U. Relative validity and reproducibility of a parent-administered semi-quantitative FFQ for assessing food intake in Danish children aged 3–9 years. Public Health Nutr. 2016;19:1184–1194. doi: 10.1017/S136898001500275X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippi A.R., Amodio E., Napoli G., Breda J., Bianco A., Jemni M. The web-based ASSO-food frequency questionnaire for adolescents: Relative and absolute reproducibility assessment. Nutr. J. 2014;13:119–129. doi: 10.1186/1475-2891-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Øverby N.C., Johannesen E., Jensen G., Skjaevesland A.-K., Haugen M. Test-retest reliability and validity of a web-based food-frequency questionnaire for adolescents aged 13-14 to be used in the Norwegian Mother and Child Cohort Study (MoBa) Food Nutr. Res. 2014;58:23956–23966. doi: 10.3402/fnr.v58.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeedi P., Skeaff S.A., Wong J.E., Skidmore P.M. Reproducibility and Relative Validity of a Short Food Frequency Questionnaire in 9-10 Year-Old Children. Nutrients. 2016;8:271. doi: 10.3390/nu8050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vereecken C.A., Maes L. A Belgian study on the reliability and relative validity of the Health Behaviour in School-Aged Children food-frequency questionnaire. Public Health Nutr. 2003;6:581–588. doi: 10.1079/PHN2003466. [DOI] [PubMed] [Google Scholar]
- 37.Vioque J., Gimenez-Monzo D., Navarrete-Muñoz E.M., Garcia-de-la-Hera M., Gonzalez-Palacios S., Rebagliato M. Reproducibility and validity of a food frequency questionnaire designed to assess diet in children aged 4-5 years. PLoS ONE. 2016;11:e0167338. doi: 10.1371/journal.pone.0167338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong J.E., Parnell W.R., Black K.E., Skidmore P.M. Reliability and relative validity of a food frequency questionnaire to assess food group intakes in New Zealand adolescents. Nutr. J. 2012;11:65–73. doi: 10.1186/1475-2891-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanfer A., Hebestreit A., Ahrens W., Krogh V., Sieri S., Lissner L. Reproducibility of food consumption frequencies derived from the children’s eating habits questionnaire used in the IDEFICS study. Int. J. Obes. 2011;35:S61–S68. doi: 10.1038/ijo.2011.36. [DOI] [PubMed] [Google Scholar]
- 40.Handeland K., Kjellevold M., Markhus M.W., Graff I.E., Frøyland L., Lie Ø. A diet score assessing Norwegian adolescents’ adherence to dietary recommendations—Development and test-retest reproducibility of the score. Nutrients. 2016;8:467. doi: 10.3390/nu8080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kant A.K. Indexes of overall diet quality: A review. J. Am. Diet. Assoc. 1996;96:785–791. doi: 10.1016/S0002-8223(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 42.McPherson R.S., Hoelscher D.M., Alexander M., Scanlon K.S., Serdula M.K. Dietary assessment methods among school-aged children: Validity and reliability. Prev. Med. 2000;31:S11–S33. doi: 10.1006/pmed.2000.0631. [DOI] [Google Scholar]
- 43.Kourlaba G., Kondaki K., Grammatikaki E., Roma-Giannikou E., Manios Y. Diet quality of preschool children and maternal perceptions/misperceptions: The GENESIS study. Public Health. 2009;123:738–742. doi: 10.1016/j.puhe.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Huybrechts I., Vereecken C., De Bacquer D., Vandevijvere S., Van Oyen H., Maes L. Reproducibility and validity of a diet quality index for children assessed using a FFQ. Br. J. Nutr. 2010;104:135–144. doi: 10.1017/S0007114510000231. [DOI] [PubMed] [Google Scholar]
- 45.Wilson A.M., Magarey A.M., Mastersson N. Reliability and relative validity of a child nutrition questionnaire to simultaneously assess dietary patterns associated with positive energy balance and food behaviours, attitudes, knowledge and environments associated with healthy eating. Int. J. Behav. Nutr. Phys. Act. 2008;5:5–16. doi: 10.1186/1479-5868-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turconi G., Celsa M., Rezzani C., Biino G., Sartirana M.A., Roggi C. Reliability of a dietary questionnaire on food habits, eating behaviour and nutritional knowledge of adolescents. Eur. J. Clin. Nutr. 2003;57:753–763. doi: 10.1038/sj.ejcn.1601607. [DOI] [PubMed] [Google Scholar]
- 47.Corder K., Crespo N.C., van Sluijs E.M., Lopez N.V., Elder J.P. Parent awareness of young children’s physical activity. Prev. Med. 2012;55:201–205. doi: 10.1016/j.ypmed.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebremariam M.K., Totland T.H., Andersen L.F., Bergh I.H., Bjelland M., Grydeland M. Stability and change in screen-based sedentary behaviours and associated factors among Norwegian children in the transition between childhood and adolescence. BMC Public Health. 2012;12:104–112. doi: 10.1186/1471-2458-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lien N., Bjelland M., Bergh I.H., Grydeland M., Anderssen S.A., Ommundsen Y. Design of a 20-month comprehensive, multicomponent school-based randomised trial to promote healthy weight development among 11–13 year olds: The Health in Adolescents study. Scand. J. Public Health. 2010;38:38–51. doi: 10.1177/1403494810379894. [DOI] [PubMed] [Google Scholar]
- 50.Andersen A., Krølner R., Currie C., Dallago L., Due P., Richter M. High agreement on family affluence between children’s and parents’ reports: International study of 11-year-old children. J. Epidemiol. Community Health. 2008;62:1092–1094. doi: 10.1136/jech.2007.065169. [DOI] [PubMed] [Google Scholar]
- 51.Brettschneider A.-K., Ellert U., Schaffrath Rosario A. Comparison of BMI derived from parent-reported height and weight with measured values: Results from the German KiGGS study. Int. J. Environ. Res. Public Health. 2012;9:632–647. doi: 10.3390/ijerph9020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huybrechts I., Himes J.H., Ottevaere C., De Vriendt T., De Keyzer W., Cox B. Validity of parent-reported weight and height of preschool children measured at home or estimated without home measurement: A validation study. BMC Pediatr. 2011;11:63–70. doi: 10.1186/1471-2431-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niedźwiedzka E., Wądołowska L., Słowińska M., Cichon R., Przybyłowicz K. Self-reported and measured values of body weight and height vs. evaluation of the nutritional status of youth. Pol. J. Environ. Stud. 2006;15:716–722. [Google Scholar]
- 54.Statistics Poland Regions of Poland. Warsaw, Poland. [(accessed on 18 December 2018)];2016 Available online: https://stat.gov.pl/obszary-tematyczne/inne-opracowania/miasta-wojewodztwa/regiony-polski-2016,6,10.html.
- 55.Field A.E., Peterson K.E., Gortmaker S.L., Cheung L., Rockett H., Fox M.K. Reproducibility and validity of a food frequency questionnaire among fourth to seventh grade inner-city school children: Implications of age and day-to-day variation in dietary intake. Public Health Nutr. 1999;2:293–300. doi: 10.1017/S1368980099000397. [DOI] [PubMed] [Google Scholar]
- 56.Ortiz-Andrellucchi A., Henríquez-Sánchez P., Sánchez-Villegas A., Peña-Quintana L., Mendez M., Serra-Majem L. Dietary assessment methods for micronutrient intake in infants, children and adolescents: A systematic review. Br. J. Nutr. 2009;102:S87–S117. doi: 10.1017/S0007114509993163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.