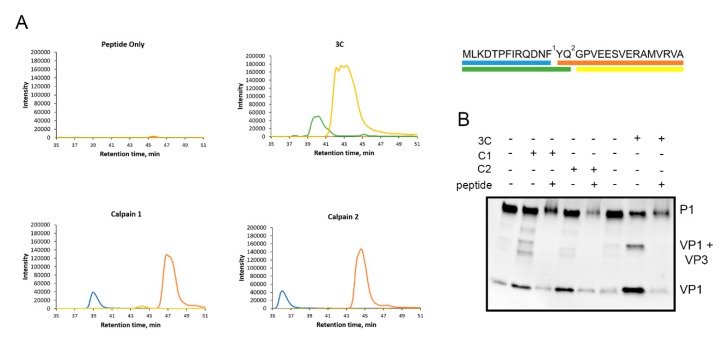
Figure 5.
Calpains cleave specifically between VP1 and VP3. A 30-amino-acid artificial peptide around the VP1-VP3 cleavage site was used to study the cleavage site of calpains in more detail. (A) The peptide was incubated with calpain 1 or calpain 2 for 2 h at +25 °C or with 3C for 20 h at RT. Also, a control reaction with no proteases was included. The cleavage products were analyzed with SWATH-MS. The peaks correspond to different cleavage products, of which the sequences are color coded in the schematic image of the peptide on the right. Numbers 1 and 2 indicate the cleavage sites of calpains and 3C, respectively. (B) P1 construct was incubated with calpain 1 (C1) or 2 (C2) for 2 h at +25 °C or with 750 ng of 3C protease for 17 h at 22 °C with or without 100 µg of the peptide. Also, controls with no proteases were included. Representative images of three experiments are shown.