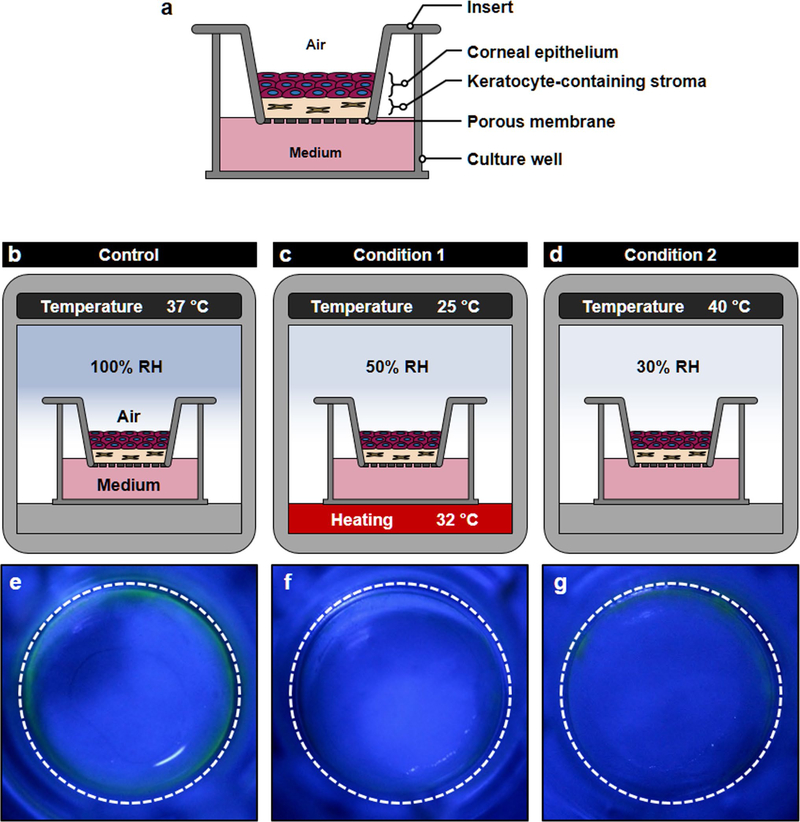
Extended Data Fig. 2 |. Responses of transwell dry-eye model to desiccating stress.
The capacity of conventional in vitro platforms to model dry eye was investigated using air-liquid interface (ALI) culture of primary human corneal epithelial cells and keratocytes in Transwell inserts. a, This in vitro model was constructed as a Transwell equivalent of the eye model by creating a thin layer of collagen hydrogel interspersed with keratocytes on the porous membrane of the insert and then plating corneal epithelial cells on the surface of the hydrogel layer. Before induction of dry eye, the tissue construct was cultured submerged for 3 d and then maintained at the ALI for another 10 d to induce differentiation and stratification of the epithelium. b–d, Simulation of evaporative dry eye in the Transwell model. b, (Control) The tissue was maintained in a regular humidified cell culture incubator (37 °C air, 100% RH). c, (Condition 1) The tissue constructs were moved to the DED simulation chamber to expose them under the same condition used for modeling dry eye in the eye model (25 °C air, 32 °C for culture medium and 50% RH). d, (Condition 2) Desiccating culture conditions previously reported in Transwell-based in vitro models of evaporative dry eye (40 °C air, 30% RH) were used. e–g, Evaluation of the response of the Transwell dry-eye models to the desiccating environment using fluorescein staining after 4 d exposure. In the Control group (e), no fluorescence was detected in the central regions of the epithelium when treated with fluorescein. Similarly, the ocular surface tissues produced in Conditions 1 (f) and 2 (g) showed the absence of fluorescein staining despite their exposure to the desiccating environment. RH, relative humidity.