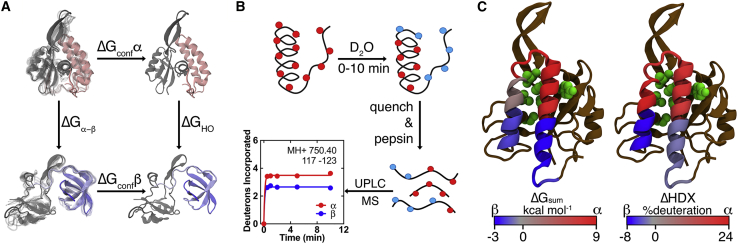
Figure 1.
Computational and experimental assessment of local stability in the metamorphic protein RfaH. (A) Shown is the thermodynamic cycle of the confinement MD approach (25) used to estimate the per-residue ΔG between both RfaH folds. The autoinhibited form with the CTD in the α-state (pink, PDB: 5OND) and the active form with the CTD in the refolded β-state (light blue, PDB: 6C6S) are confined toward a deeply minimized state through a harmonic constraint (ΔGconf), allowing calculation of the difference in free energy between these structures (ΔGHO). (B) Shown is a scheme of HDXMS experiments (52). Both full-length RfaH and the isolated CTD were incubated in deuterated buffer for different reaction times, quenched, and pepsin digested for analyzing the local extent of deuteron incorporation. (C) Shown are cartoon representations of the full-length αRfaH, in which the CTD covers the RNAP-binding residues from the NTD (green), summarizing our findings from simulations (left) and experiments (right) on the differential local stability toward the α- (red) and β-state (blue) of the CTD. To see this figure in color, go online.