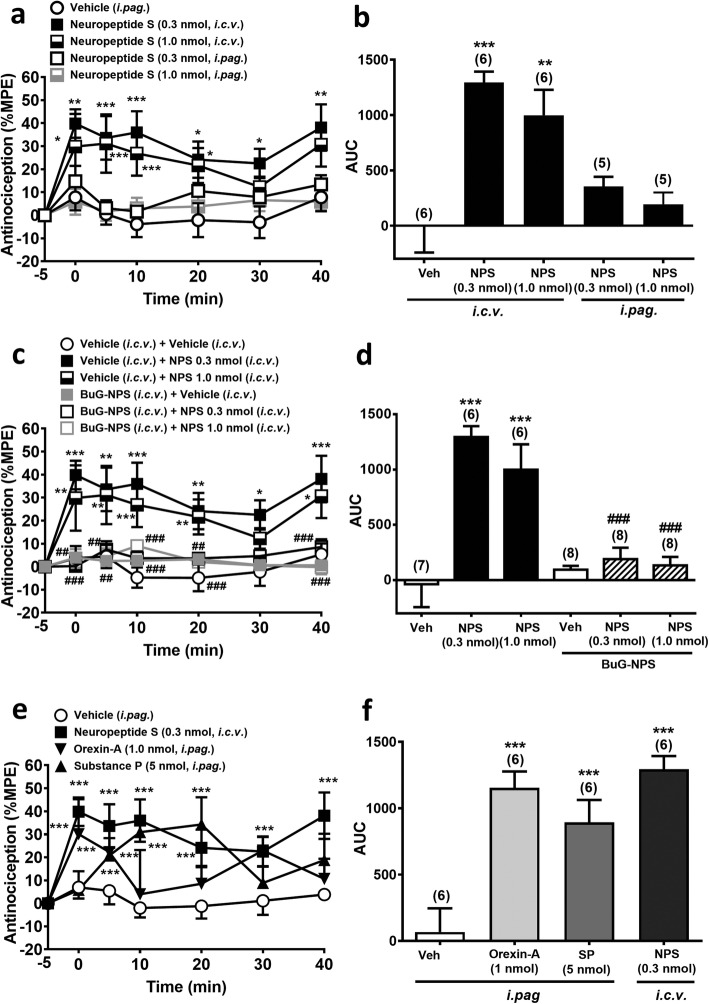
Fig. 2.
Antinociceptive effects induced by NPS, orexin-A and substance P in the mouse hot-plate test. a-b: Antinociceptive effects of NPS (0.3 & 1.0 nmol) by i.c.v. or i.pag. microinjection. c-d: Antinociceptive effects of i.c.v. NPS challenged by an NPSR antagonist, [tBu-D-Gly5] NPS (10 nmol, i.c.v.). e-f: A comparison of antinociceptive effects of orexin-A (1 nmol, i.pag.), substance P (5 nmol, i.pag.), and NPS (0.3 nmol, i.c.v.). a, c and e: The time course of the antinociceptive effect expressed as the percentage of the maximal possible effect (MPE) (two-way ANOVA /post hoc Bonferroni test). b, d and f: The area under the curve (AUC) of the % MPE measured within 40 min in each treatment group (one-way ANOVA /post hoc Tukey test). The number denoted in the parentheses above each bar is the n number of mice tested in each group. Data are mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the vehicle control group, ###p < 0.001 vs. NPS 0.3 or 1.0 group