Abstract
Background
Macrophages and T lymphocytes in the perivascular adipose tissue (PvAT) were previously linked to coronary artery disease. However, the role of these cells and B lymphocytes in the human PvAT adjacent to unstable atherosclerotic plaques has not been investigated. Moreover, previous studies were inconclusive on whether PvAT inflammation was restricted to the surroundings of the atheroma plaque.
Methods and Results
Coronary arteries were freshly dissected with the surrounding PvAT. Atherosclerotic plaques were classified according to the internationally accepted anatomopathological criteria. Immune cells in the PvAT were detected using immunohistochemistry and then quantified. We used linear and logistic regressions with robust standard errors, adjusted for possible confounding factors. In 246 atherosclerotic plaques (205 stable and 41 unstable plaques) from 82 participants (mean age=69.0±14.4 years; 50% men), the percentage of arterial obstruction was positively correlated with the densities of CD68+ macrophages (P=0.003) and CD20+ B lymphocytes (P=0.03) in the periplaque PvAT. The number of cells was greater in the periplaque PvAT than in the distal PvAT (macrophages, P<0.001; B lymphocytes, P=0.04). In addition, the density of macrophages in the periplaque PvAT was greater in the presence of unstable plaques (P=0.03) and was also greater near unstable plaques than in the distal PvAT (P=0.001). CD3+ T lymphocytes were not associated with percentage of obstruction and stable/unstable plaque composition.
Conclusions
The density of CD20+ B lymphocytes and CD68+ macrophages in periplaque PvAT was increased with plaque size, and the CD68+ macrophages were greater near unstable atherosclerotic plaques than near stable lesions. This inflammation was more intense in the periplaque PvAT than in the PvAT distal to the atherosclerotic plaques.
Keywords: atherosclerosis, B cell, coronary artery disease, inflammation, macrophages, pericoronary adipose tissue
Subject Categories: Basic Science Research, Coronary Circulation, Inflammation, Pathophysiology, Translational Studies
Clinical Perspective
What Is New?
Our study demonstrated that inflammation mediated by CD68+ macrophages and CD20+ B lymphocytes in the perivascular adipose tissue (PvAT) adjacent to the most important atherosclerotic plaque increased with the percentage of arterial obstruction in the coronary arteries.
We are the first to demonstrate that the density of C68+ macrophages in the PvAT adjacent to unstable plaques was greater than in the PvAT adjacent to stable plaques.
Inflammation was more intense in the PvAT adjacent to stenotic and acute lesions than in the adipose tissue distal of lesions and without atherosclerosis.
What Are the Clinical Implications?
Basic research using human samples of PvAT adjacent to coronary arteries with and without atherosclerosis can expand the findings from animal models on the associations between specific immune cells and atherosclerotic plaque size and composition.
These studies are important to better determine potential targets for the development of new drugs in the different stages of the atherosclerotic process.
Introduction
Although the frequency of coronary heart disease has declined recently, it remains the leading cause of mortality1 and disability worldwide.2 The main cause of coronary heart disease is atherosclerosis.3 The immune system has a central role in the development of coronary artery disease (CAD).4 Therefore, some clinical trials have investigated the role of anti‐inflammatory agents to treat patients with CAD. In JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), statin therapy was highly effective in decreasing major adverse cardiovascular events in individuals with low‐density lipoproteins and high levels of high‐sensitivity CRP (C‐reactive protein), suggesting an anti‐inflammatory action of rosuvastatin.5 In CANTOS (Canakinumab Anti‐Inflammatory Thrombosis Outcome Study), therapy with monoclonal antibody target at interleukin‐1β (canakinumab), which is involved in the interleukin‐6 signaling, significantly reduced major adverse cardiovascular events independent of lipid levels. However, patients who used canakinumab had more deaths secondary to infections, especially in older adults and patients with diabetes mellitus.6
In addition, the perivascular adipose tissue (PvAT) has been reported as a potential trigger for atherosclerosis, independent of traditional approaches, where CAD initiates from endothelial cells and the intima layer.7 In experimental studies, PvAT transplanted in low‐density lipoprotein–deficient knockout mice promoted endothelial dysfunction and atherosclerosis in aortic and thoracic aorta.8 PvAT also showed increased MCP‐1 (monocyte chemoattractant protein‐1) levels after transplantation of perivascular visceral adipose tissue adjacent to carotid arteries, causing large lipid‐rich atherosclerotic lesions, high macrophage density, and fibrin deposits compared with mice transplanted with subcutaneous adipose tissue.9 However, extrapolation of these results for humans is difficult because of differences between human and animal models, including characteristics of atherosclerotic plaque.10 Translational studies from experimental to human have reported that inflammation in the epicardial adipose tissue (EAT) and in the PvAT was associated with CAD evaluated by biopsy,11, 12, 13 autopsy,14, 15, 16, 17 and cardiac imaging.14, 18, 19 Most studies investigated the role of CD68+ macrophages in the EAT11, 12, 13 and PvAT15, 16, 17 on stable CAD. The number of CD68+ macrophages in the PvAT was also correlated with the number of immune cells in the atherosclerotic plaques.15, 16, 17
CD3+ T lymphocytes in the PvAT were identified only in studies using biopsy samples.11 The subtype CD8+ T lymphocytes in the EAT were more frequent in patients with CAD than in controls, whereas the density of the CD4+ T lymphocytes present in the EAT was similar between CAD and control groups.12 The role of B lymphocytes in the PvAT adjacent to the atherosclerotic plaques remains unknown.20 CD20+ B lymphocytes secreted cytokines, which increased the vulnerability of the atherosclerotic plaques by the recruitment of macrophages and increased the necrotic core in advanced lesions.21 Moreover, CD20+ B lymphocytes produced and secreted antibodies against antigens in the atherosclerosis plaque, such as β‐2 glycoprotein I, heat shock proteins (HSP),22 and self‐modified antigens.23 The CD20+ B lymphocytes can also present antigens to T lymphocytes, which secrete cytokines and promote a proinflammatory response.24 Indeed, the association of these immune cells with unstable atherosclerotic plaques has not been investigated. In addition, it is unknown whether these immune cells are restricted to the region surrounding the atherosclerotic plaques. A greater inflammation density was detected around culprit lesions compared with nonculprit lesions by imaging methods,14, 18 whereas previous autopsy studies were inconclusive.16, 17 Autopsy studies investigated only macrophages in the PvAT.15, 16, 17 Although biopsy studies explored other types of immune cells,11, 13 samples obtained during cardiac surgery could initiate an inflammatory response, with an increase in monocytes and heat shock proteins, mainly with the use of extracorporeal circulation.25 Moreover, no studies to date have accounted for confounders in statistical analyses.12, 13, 14, 15, 16, 17 Therefore, we investigated the association of the density of CD68+ macrophages, CD3+ T lymphocytes, and CD20+ B lymphocytes in the periplaque PvAT with plaque composition and with the percentage of arterial obstruction. In addition, we compared the density of these immune cells in the periplaque PvAT and in the distal PvAT without atherosclerosis. We hypothesized that the density of CD68+ macrophages, CD3+ T lymphocytes, and CD20+ B lymphocytes in the PvAT adjacent to coronary artery lesions would be greater in the presence of larger arterial obstruction and that the density of these immune cells would be greater in PvAT adjacent to coronary arteries with unstable lesions in comparison with stable lesions. In addition, we supposed that the inflammation in periplaque PvAT would be greater than in distal PvAT.
Methods
This study was approved by the local ethics committee, and the next of kin (NOK) signed the informed consent before the sample collection. The data that support the findings of this study are available from the corresponding author on request.
Subjects
This cross‐sectional study included cadavers of subjects who had died from nontraumatic causes.26 The cadavers underwent full‐body autopsy at the São Paulo Autopsy Service from 2013 to 2015. Inclusion criteria were as follows: aged ≥30 years; postmortem interval <24 hours; and a NOK with at least weekly interaction with the deceased in the 6 months before death. We excluded participants with cardiovascular conditions that could induce inflammation (ie, presence of a stent, chronic or acute pericarditis, pericardial effusion, myocarditis, endocarditis, Chagas disease, hemopericardium, cardiac tamponade, and previous cardiac surgery, including transplant). We also excluded participants with signs of systemic inflammation: autoimmune diseases, use of corticosteroids, use of immunosuppressive drugs, treatment with chemotherapy or/and radiotherapy, and diagnosis of sepsis before death. Moreover, we evaluated the presence of sepsis signs in the postmortem examination. Although the sepsis diagnosis is clinical, previous studies showed that it is possible to infer its presence by postmortem anatomopathological examination.27, 28 A pathologist (L.F.F.S.) examined slides stained with hematoxylin‐eosin from participants’ lung, heart, liver, kidney, and spleen. The presence of at least 2 organs with signs of sepsis combined with focus of infection was necessary for the diagnosis of sepsis.28 For example, we excluded participants with the following: (1) neutrophil infiltration in the lung associated with pleuritis caused by bronchopneumonia (Figure S1A and S1B); (2) acute tubular necrosis in the kidney secondary to shock (Figure S1C); (3) acute splenitis (Figure S1D); and (4) bronchopneumonia with hepatic congestion and steatosis (Figure S1E). We also excluded subjects whose NOKs were unable to provide clinical information.
Collection of Coronary Arteries and PvAT
Hearts were collected and washed in running water. Agar was injected into the ostia of the coronary arteries to avoid the flatting of the coronary arteries. The right coronary, left main coronary, left anterior descending, and circumflex arteries were dissected with 10 mm of PvAT around the coronary artery trajectories. Afterwards, the PvAT was removed and identified at regular intervals of 15 mm. The coronary arteries were fixed in 4% buffered paraformaldehyde for at least 5 days. The PvAT was fixed for 24 hours.26 Samples of coronary arteries and PvAT were immersed in paraffin.
Assessment of Coronary Artery Atherosclerosis
Coronary arteries were cut at 15‐mm intervals to match the samples of PvAT. These segments were divided into sections of 5 mm each to evaluate the atherosclerotic plaques. The sections with the largest arterial obstruction or those suspected of having unstable atherosclerotic plaques were sampled from each coronary artery (periplaque PvAT).29, 30 To define the main lesion, sampling of unstable plaques was preferred over stable ones. Coronary artery sections without macroscopic lesions and at least 15 mm away from any atherosclerotic plaques also represented a control area (distal PvAT).
Afterwards, these samples were stained with hematoxylin‐eosin, Verhoeff, and Masson trichrome. The slides were photographed with the aid of a stereomicroscope (SMZ 1000; Nikon, Tokyo, Japan). We measured the areas delimited by the lumen and by the internal elastic lamina using the software ImageJ;31 and we calculated the percentage of arterial obstruction by dividing the difference of the internal elastic lamina area and lumen area by the internal elastic lamina area, and then multiplying the result by 100.32 We classified the atherosclerotic lesions according to the American Heart Association criteria.29 Stable lesions were defined by the presence of fibroatheroma, calcification, lipid core, or whole fibrous cap, whereas the plaque was considered unstable in the presence of ulceration, intraplaque hemorrhage, or total/partial thrombosis (Figures S2 and S3).29
Assessment of PvAT Inflammation
We identified macrophages (Monoclonal Mouse Anti‐Human CD68, clone KP1, 1ML Dako‐M081401, 1:5000 μL), T lymphocytes (Polyclonal Rabbit Anti‐Human CD3, 1ML Dako‐M045201, 1:1500 μL), and B lymphocytes (Monoclonal Mouse Anti‐Human CD20cy, clone L26, 1ML Dako‐M075501, 1:12000 μL) in silanized slides of periplaque and distal PvAT. The slides were scanned with a scanner (Pannoramic Scan 250 Flash III; 3DHISTECH, Hungary) and analyzed with a virtual microscope (Pannoramic Viewer software; 3DHISTECH). The cells were counted into 20 random fields of 600‐µm diameter.
Clinical Data
We collected information on sociodemographic variables (age, sex, race, and education). The deceased's medical history was determined using a semistructured interview applied to the NOK; and the medical history includes information on hypertension, diabetes mellitus, CAD, heart failure, dyslipidemia, stroke, physical activity (defined by the performance of domestic, work, or formal physical activities at least 3 times per week), current alcohol use, and current smoking.33 Body mass index was obtained by direct measurements of weight and height, with the deceased in the supine position without clothes or shoes.
Statistical Analysis
We estimated a sample size of 78 subjects, considering an effect size of 0.93 for the mean difference in immune cells between CAD and the control group,14 with power of 90% and α of 5%.
The dependent variables were the percentage of arterial obstruction (continuous variable) and plaque composition (binary variable). The independent variable was the density of the CD68+ macrophages, CD3+ T lymphocytes, and CD20+ B lymphocytes (continuous variables). The associations of cell density in the periplaque PvAT with the percentage of arterial obstruction were tested using linear regression with robust SEs to account for repeated measures in the same individual (3 arterial segments per person) using the Huber‐White sandwich estimators.34 The associations of cell density in the periplaque PvAT with plaque composition groups were tested using logistic regression with robust SEs through cluster adjustment, where the model tested the odds to unstable over stable plaques. In addition, we compared the cells in the PvAT around stable plaques and in the PvAT around unstable lesions in the same individual using Wilcoxon matched pairs rank test.
We also calculated the differences between the density of immune cells in the periplaque PvAT and the density of these cells in the distal PvAT. We then tested the association of these differences with the percentage of arterial obstruction and plaque composition using linear and logistic regressions with robust SEs, respectively. All the models were adjusted for potential confounders (age,35, 36, 37, 38, 39, sex40, 41, hypertension,42, 43, 44 diabetes mellitus,42, 45 body mass index,42, 46, 47 smoking,42, 48, physical inactivity,49, 50 and alcohol use). The significance level was 5%. The statistical software used was Stata 13.0 (Stata Corp, TX).
Results
We included 82 participants (mean age=69.0±14.4 years; 50% men; 58% white; and 67% had <4 years of education). Hypertension (74%) and diabetes mellitus (37%) were most frequent risk factors in our sample. Among participants with hypertension, 55% used antihypertensive drugs; and among those with diabetes mellitus, 29% used hypoglycemic drugs and 8% used insulin (Table 1). Most NOK (85.3%) had daily interaction with the deceased. The most advanced lesions in each coronary artery were represented, which resulted in 246 coronary artery samples. In 54 participants with only stable lesions, we found 162 lesions. In 28 individuals with both stable and unstable lesions, we found 41 unstable lesions and 43 stable lesions (Figure S4). The mean percentage of arterial obstruction of proximal lesions was 56.7±26.0%; and of the distal fragments, 17.3±10.3% (Table S1). We tested the correlation between the sociodemographic and clinical data with the density of immune cells and found that age and body mass index correlated with the density of CD68+ macrophages (Table S2). In addition, physical inactivity correlated with CD3+ T lymphocytes, and no variable correlated with CD20+ B lymphocytes (Tables S3 and S4).
Table 1.
Characteristics of the Sample (n=82)
| Variables | Value |
|---|---|
| Age, y | 69.0 (14.4) |
| Women | 41 (50) |
| White | 48 (58) |
| Education, median (IQR), y | 4 (1–6) |
| Hypertension | 61 (74) |
| Diabetes mellitus | 30 (36) |
| Coronary artery disease | 10 (12) |
| Heart failure | 19 (23) |
| Stroke | 10 (12) |
| Dyslipidemia | 14 (17) |
| Body mass index, kg/m2 | 24.4 (5.8) |
| Physical inactivity | 56 (68) |
| Smoking | 12 (15) |
| Alcohol use | 24 (29) |
| Antihypertensive drugs | 45 (55) |
| Hypoglycemic drugs | 24 (29) |
| Insulin | 7 (8) |
| Lipid‐lowering drugs | 6 (7) |
| Postmortem interval, h | 15.6 (3.1) |
Data are given as mean (SD) or number (percentage), unless otherwise indicated. IQR indicates interquartile range.
CD68+ Macrophages
The density of CD68+ macrophages in the periplaque PvAT was positively correlated to the percentage of coronary artery obstruction in the multivariable analysis (β=2.06; 95% CI=0.71–3.41; P=0.003) (Table 2, Figure 1, and Figure 2A). The difference between the density of CD68+ macrophages in the periplaque PvAT and in the distal PvAT was related to the percentage of coronary artery obstruction (β=2.85; 95% CI=1.32–4.39; P<0.001) (Table 2, Figure 2C). In addition, the density of CD68+ macrophages in the periplaque PvAT was greater near unstable plaques than near stable plaques (odds ratio=1.15; 95% CI=1.01–1.31; P=0.03) (Table 2, Figure 2B). This finding persisted when we compared the stable and unstable lesions in the same individual (P=0.008) (Table S5). Macrophages were more frequent in the periplaque PvAT than in the distal PvAT in the presence of unstable plaques (odds ratio=1.77; 95% CI=1.28–2.47; P=0.001) (Table 2, Figure 2D).
Table 2.
Association Between Immune Cells and Coronary Artery Atherosclerosis (n=246 Artery Segments)
| Immune Cell Density | Univariate Model | Adjusted Modela | ||||
|---|---|---|---|---|---|---|
| CD68+ macrophages | ||||||
| % of obstruction | β | 95% CI | P Value | β | 95% CI | P Value |
| PvATp (10−5cells/μm2)b | 2.19 | 0.88 to 3.51 | 0.001 | 2.06 | 0.71 to 3.41 | 0.003 |
| Difference: PvATp and PvATd (10−5cells/μm2)b | 2.30 | 0.57 to 4.03 | 0.01 | 2.85 | 1.32 to 4.39 | <0.001 |
| Unstable plaque composition | OR | 95% CI | P Value | OR | 95% CI | P Value |
| PvAT (10−5cells/μm2)c | 1.12 | 1.02 to 1.25 | 0.02 | 1.15 | 1.01 to 1.31 | 0.03 |
| Difference: PvATp and PvATd (10−5cells/μm2)c | 1.42 | 1.14 to 1.78 | 0.002 | 1.77 | 1.28 to 2.47 | 0.001 |
| CD3+ T lymphocytes | ||||||
| % of obstruction | β | 95% CI | P Value | β | 95% CI | P Value |
| PvATp (10−5cells/μm2)b | 3.12 | −1.17 to 7.41 | 0.15 | 3.42 | −0.85 to 7.69 | 0.11 |
| Difference: PvATp and PvATd (10−5cells/μm2)b | 1.78 | −2.18 to 5.74 | 0.37 | 2.15 | −1.83 to 6.13 | 0.28 |
| Unstable plaque composition | OR | 95% CI | P Value | OR | 95% CI | P Value |
| PvATp (10−5cells/μm2)c | 1.31 | 0.92 to 1.87 | 0.13 | 1.37 | 0.94 to 2.00 | 0.09 |
| Difference: PvATp and PvATd (10−5cells/μm2)c | 1.49 | 0.85 to 2.59 | 0.15 | 1.55 | 0.77 to 3.11 | 0.21 |
| CD20+ B lymphocytes | ||||||
| % of obstruction | β | 95% CI | P Value | β | 95% CI | P Value |
| PvAT (10−5cells/μm2)b | 40.10 | 7.02 to 73.19 | 0.01 | 36.38 | 1.83 to 70.93 | 0.03 |
| Difference: PvATp and PvATd (10−5cells/μm2)b | 37.66 | 3.13 to 72.18 | 0.03 | 39.49 | 1.89 to 77.09 | 0.04 |
| Unstable plaque composition | OR | 95% CI | P Value | OR | 95% CI | P Value |
| PvAT (10−5cells/μm2)c | 26.91 | 1.38 to 523.65 | 0.03 | 23.44 | 0.67 to 812.92 | 0.08 |
| Difference: PvATp and PvATd (10−5cells/μm2)c | 13.39 | 0.54 to 332.03 | 0.11 | 8.56 | 0.16 to 447.22 | 0.28 |
OR indicates odds ratio; PvATd, distal perivascular adipose tissue; PvATp, periplaque perivascular adipose tissue.
Adjusted for age, hypertension, diabetes mellitus, body mass index, smoking, alcohol use, and physical inactivity.
Linear regression with robust SEs to account for repeated measures in the same individual through cluster adjustment.
Logistic regression with robust SEs to account for repeated measures in the same individual through cluster adjustment.
Figure 1.
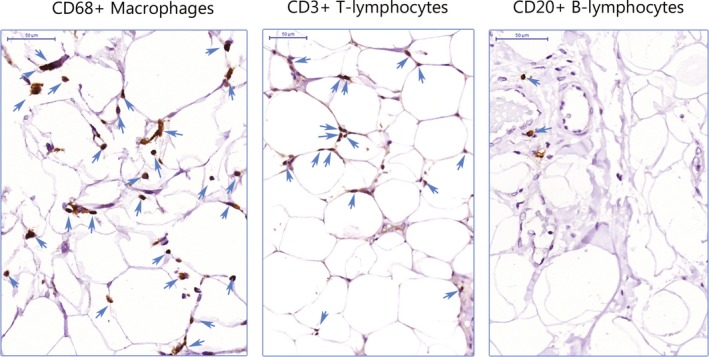
CD68+ macrophages, CD3+ T lymphocytes, and CD20+ B lymphocytes. Immune cells stained in brown and marked with a blue arrow infiltrated into the perivascular adipose tissue (bar=50 µm).
Figure 2.
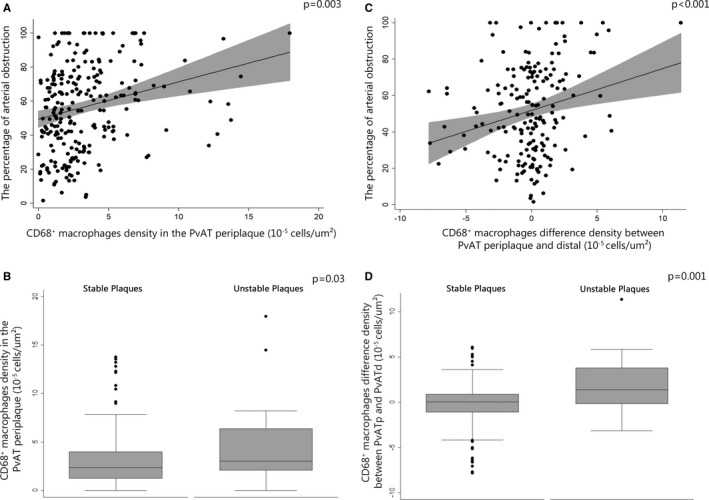
Association between CD68+ macrophages and atherosclerosis. A, Scatterplot of the density of macrophages in periplaque perivascular adipose tissue (PvAT) with the percentage of arterial obstruction. B, Box plot of the density of macrophages in periplaque PvAT in stable and unstable plaque groups. C, Scatterplot of the difference of the macrophage density in periplaque PvAT and distal PvAT with the percentage of arterial obstruction. D, Box plot of the difference of macrophage density between periplaque PvAT and distal PvAT in stable and unstable plaque groups.
CD3+ T Lymphocytes
The density of CD3+ T lymphocytes in the periplaque PvAT was not associated with percentage of obstruction, nor with plaque composition. Moreover, there was no difference in the cell density between the periplaque PvAT and the distal PvAT (Table 2, Figure 1, Table S5, and Figure S5).
CD20+ B Lymphocytes
The percentage of arterial obstruction increased with the density of CD20+ B lymphocytes in the periplaque PvAT (β=36.38; 95% CI=1.83–70.93; P=0.03), and the density of B cells in the periplaque PvAT was greater compared with distal PvAT (β=39.49; 95% CI=1.89–77.09; P=0.04) (Table 2, Figures 1 and 3A). We did not find differences in the B‐lymphocyte densities in the periplaque PvAT around unstable plaques compared with the PvAT around stable plaques (odds ratio=23.44; 95% CI=0.67–812.92; P=0.08) (Table 2, Figure 3B). The B‐lymphocyte densities in the PvAT were similar around stable and unstable lesions in the same individual (P=0.58) (Table S5). In addition, the B‐lymphocyte densities near unstable lesions were similar in the periplaque and distal PvAT (odds ratio=8.56; 95% CI=0.16–447.22, P=0.28) (Table 2, Figure 1, Figure 3A, 3C and 3D).
Figure 3.
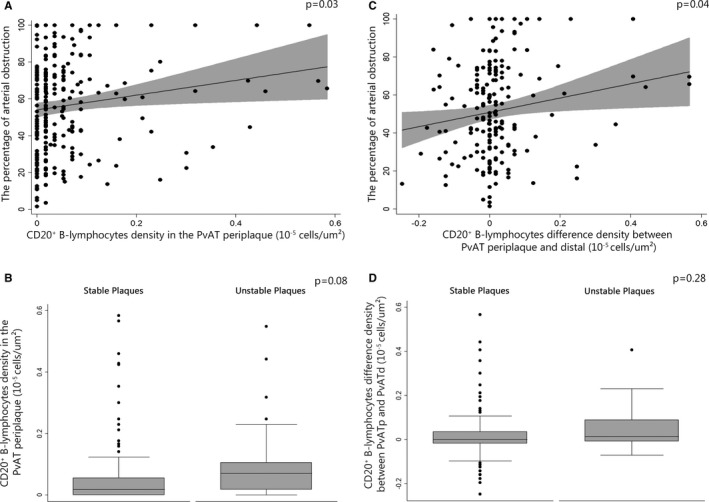
Association between CD20+ B lymphocytes and atherosclerosis. A, Scatterplot of the density of B lymphocytes in periplaque perivascular adipose tissue (PvAT) with the percentage of arterial obstruction. B, Box plot of the density of B lymphocytes in periplaque PvAT in stable and unstable plaque groups. C, Scatterplot of the difference of the B‐lymphocyte density in periplaque PvAT and distal PvAT with the percentage of arterial obstruction. D, Box plot of the difference in density of B lymphocytes between periplaque PvAT and distal PvAT in stable and unstable plaque groups.
Discussion
We found that the densities of CD68+ macrophages and CD20+ B lymphocytes in the periplaque PvAT were greater in the presence of larger luminal obstruction by atherosclerotic plaques. In addition, the density of CD68+ macrophages was greater around unstable lesions than around stable lesions. These findings were more prominent in the PvAT surrounding the most critical atherosclerotic lesions compared with the PvAT near artery regions without macroscopic lesions and distant from the culprit lesions.
CD68+ Macrophages and CAD
The positive correlation between the density of CD68+ macrophages and the size of the atherosclerotic plaques was previously described.16 Infiltrations of CD68+ macrophages in the PvAT were similar between patients with dilated cardiomyopathy and patients with CAD.17 Previous studies demonstrated an association of chronic CAD with CD68+ macrophages and polarized macrophages.12, 13, 15, 16 However, these studies used EAT samples obtained during surgery, where the collection of the sample adjacent to the most important lesion may be difficult.12, 13 This is important because the EAT transcriptomic signature varied according to the anatomical region of the PvAT sampling.51 In autopsy studies,15, 16 CD68+ macrophages were associated with stable plaque composition.15 However, we were the first to show in humans that CD68+ macrophage density in the PvAT adjacent to unstable coronary lesions was greater than near stable lesions. The association between unstable lesions and macrophages was only investigated in samples of carotid arteries and aorta, where the number of polarized macrophages inside the arterial wall was greater in unstable plaques.52 This finding suggested that macrophages may be involved with the rupture of the fibrous cap and the progression of disease severity.52
In addition, the association between the density of CD68+ macrophages with plaque size and unstable atherosclerotic plaques was greater in the periplaque PvAT than distal PvAT in the same individual, which emphasizes the potential intrinsic role of CD68+ macrophages in CAD development. This result is in line with imaging studies, which found more inflammation around the culprit lesion than near nonculprit lesions.14, 19 In the only autopsy study that examined this association, no difference was found in the density of macrophages in periplaque and distal pvAT,16 probably because of differences in sample size and distal PvAT sampling. Our result that inflammation is not equally distributed in the EAT is expected because macrophages are cytokine‐secreting phagocytic cells that recruit other cells to the site of inflammation.53 Therefore, macrophages in the PvAT could have an effective role in the atherogenesis severity in humans.
CD3+ T Lymphocytes and CAD
We did not find any difference in the density of CD3+ T lymphocytes, which are a general marker for T lymphocytes,54 between stable and unstable plaque groups, or any correlation between the density of these immune cells and the percentage of arterial obstruction. However, our results cannot be compared directly with previous studies. Although a greater infiltrate of CD3+ T lymphocytes, CD68+ macrophages, and mast cells was found in the EAT, the assessment was qualitative and only in obese individuals.11 Hirata et al found a greater number of CD8+ T lymphocytes in PvAT in patients with CAD compared with a control group.12
CD20+ T Lymphocytes and CAD
The density of CD20+ B lymphocytes in the PvAT was positively correlated with the percentage of arterial obstruction, but this density was similar between stable and unstable plaques. The relationship of these cells in PvAT adjacent to atherosclerotic plaques has not been yet investigated. Our results suggest that CD20+ B lymphocytes could play a role in CAD progression.22 On the other hand, macrophages and T cells seem to play an essential role since the early stages of atherosclerosis development.22 We used the CD20 marker, which was related to atherosclerosis progression in animal models.55, 56 Treatments with anti‐CD20 attenuated the atherosclerosis expression.55, 56 The transfer of CD20+ cells to lymphocyte‐deficient mice increased the plaque size and macrophage accumulation in these lesions.56 In addition, we did not find any difference in the density of B lymphocytes between stable and unstable atherosclerotic plaques. This finding may caused by the short time interval between the acute coronary event and the individual's time of death. Activated B cells may not have had enough time to complete the process of recruitment, differentiation, and migration to unstable plaque site. In mice models, an increase in B‐lymphocyte number in the heart muscle was only present after 5 days of myocardial infarct induction.57 More studies should be conducted in experimental models to determine the response time of B‐lymphocyte infiltration in the PvAT after acute coronary artery lesions.
Can Inflammation in the Periplaque PvAT Promote Coronary Atherosclerosis?
Periplaque PvAT may promote atherosclerosis3 through 2 possible mechanisms. First, the PvAT can communicate with the atherosclerotic plaque through the infiltration of adipocytes in the adventitial layer,58 as we observed in our study. Second, the vasa vasorum that infiltrates the PvAT could bring the inflammatory cells from the PvAT to the coronary artery.
The question of whether the inflammation in PvAT is cause or consequence of atherosclerosis remains unknown. A recent experimental study using apolipoprotein E–deficient mice suggested that inflammation in the PvAT precedes atherosclerotic plaque formation.59 Moreover, the infiltration of leukocyte in the PvAT was greater than in the vessel wall,59 and the PvAT expresses genes associated with the production of complex lipids, which are also present in atherosclerosis.51, 60 Macrophages containing lipoproteins were found only in the periplaque PvAT, suggesting that these macrophages carried the lipoproteins from the PvAT to the artery wall.60 Although these experimental lines of evidence point to the causality direction from the PvAT to coronary artery wall, a complex bidirectional communication of inflammatory cells between the arterial wall and the PvAT may be present.61
Limitations and Strengths
Our study has several strengths. We investigated the association of B lymphocytes and T lymphocytes in human PvAT with coronary atherosclerosis using autopsy material. Basic research using human samples is scarce because of the decreased number of autopsies performed worldwide. In addition, we compared the density of these cells in unstable plaques, and no previous study has performed analyses with adjustment for confounders. However, some limitations should be considered. First, it is possible that a systemic inflammatory process could be triggered by the autopsy procedure. However, the stability of the cell counts is unknown in autopsy studies. Nonetheless, we excluded subjects with sepsis diagnosis, as well as other cardiac or systemic inflammatory conditions. In addition, the postmortem interval of all samples was <24 hours (mean postmortem interval=15.6 hours), which probably limited the inflammatory process after death.62 Moreover, previous studies used samples obtained during surgery,11, 12, 13 which probably triggers more intense inflammatory responses than the autopsy procedure, mainly when extracorporeal circulation is used.25, 63 Second, we did not measure serum levels of inflammation and could not adjust our analyses for the presence of this important variable. Third, we cannot establish relations of causality because this is a cross‐sectional study. Finally, we did not follow participants during life, and clinical variables were reported by the NOK. To minimize bias, we only included reliable NOKs. Using this approach, our interview showed high accuracy in previous studies.32, 33, 64, 65 Future research in human samples will be necessary to show if specific types of macrophages, B lymphocytes, and T lymphocytes with proatherogenic or atheroprotective roles in the PvAT may have different associations with atherosclerosis pathogenesis.20
In conclusion, a greater density of macrophages and B lymphocytes in the periplaque PvAT was associated with an increase in atherosclerotic obstruction. Moreover, the density of CD68+ macrophages was greater in the periplaque PvAT near unstable plaques. Finally, the number of macrophages and B lymphocytes was higher in the PvAT surrounding critical plaques than in segments of PvAT without atherosclerosis.
Sources of Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (2013/00335‐2, 2013/12290‐3) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (1074888).
Disclosures
Dr Sommer Bittencourt received research support from Sanofi and speaker fees from Boston Scientific for work not related to this study. The remaining authors have no disclosures to report.
Supporting information
Table S1. Descriptive Analysis of Coronary Artery Atherosclerosis and Immune Cells in the Perivascular Adipose Tissue
Table S2. Association of Density of CD68+ Macrophages in the PvAT With Sociodemographic and Clinical Data (n=246)
Table S3. Association of Density of CD3+ T‐Lymphocytes in the PvAT with Sociodemographic and Clinical Data (n=246)
Table S4. Association of Density of CD20+ B‐Lymphocytes in the PvAT with Sociodemographic and Clinical Data (n=246)
Table S5. Comparison of Immune Cells Between Perivascular Adipose Tissue of Unstable Atherosclerosis and Perivascular Adipose Tissue of Stable Atherosclerosis in the Same Individual (n=26 matched pairs)
Figure S1. Signs of sepsis detected during the anatomopathological post‐mortem exam. A, microscopic photograph (×10 magnification) of lung with neutrophil infiltration due to bronchopneumonia. B, microscopic photograph (×40 magnification) of neutrophil infiltrate in the lung. C, microscopic photograph (×40 magnification) acute tubular necrosis responsive to shock in the kidney; D, microscopic photograph (×10 magnification) of acute splenitis; E, microscopic photograph (×10 magnification) of hepatic congestion and steatosis secondary to bronchopneumonia.
Figure S2. Coronary artery with intraplaque hemorrhage. A, Coronary artery fragment stained with haematoxylin‐eosin (×2 of magnification). I: erythrocytes (×40 of magnification); II: lipid core (×40 of magnification); III: narrowing of the fibrous cap adjacent to the intraplaque hemorrhage (×40 ofmagnification). B, Coronary artery fragment stained with Masson's trichrome (×2 ofmagnification). I: erythrocytes (×40 of magnification); II: lipid core (×40 of magnification); III: narrowing of fibrous cap adjacent to intraplaque hemorrhage (×40 of magnification); IV: lipid core and intraplaque hemorrhage (×10 of magnification)and V: fibroses (×10 of magnification).
Figure S3. Coronary artery with thrombus. A,Coronary artery fragment stained with haematoxylin‐eosin (×2 of magnification). I: lipidcore (×40 of magnification); II: erythrocytes inside the thrombus (×40 of magnification); III: calcification (×40 of magnification). B, Coronary artery fragment stained with Masson'strichrome (×2 of magnification). I: Periplaque perivascular adipose tissue near the adventitialayer (×10 of magnification); II: vasa vasorum (×10 of magnification); III: thrombus (×40 of magnification).
Figure S4. Flowchart of study participants. HIV indicates Human Immunodeficiency Virus; SPAS, São Paulo Autopsy Service.
Figure S5. Association between CD3+ T‐lymphocytes and atherosclerosis. PvATp, periplaque perivascular adipose tissue; PvATd, distal perivascular adipose tissue. A, Scatter plot of the density of CD3+ T‐lymphocytes in periplaque PvAT with the percentage of coronary artery obstruction. B, Box plot graph of the density of CD3+ T‐lymphocytes in periplaque PvAT by stable and unstable plaque groups. C, Scatter plot of the difference of the CD3+ T‐lymphocytes density in periplaque PvAT and distal PvAT with the percentage of coronary artery obstruction. D, Box plot graph of the difference density of CD3+ T‐lymphocytes between periplaque PvAT and distal PvAT by stable and unstable plaque groups. PvATd indicates distal perivascular adipose tissue; PvATp, periplaque perivascular adipose tissue.
(J Am Heart Assoc. 2019;8:e013793 DOI: 10.1161/JAHA.119.013793.)
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019; 39:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 4. Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated c‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 7. Blomkalns AL, Chatterjee T, Weintraub NL. Turning ACS outside in: linking perivascular adipose tissue to acute coronary syndromes. Am J Physiol Circ Physiol. 2010;298:H734–H735. [DOI] [PubMed] [Google Scholar]
- 8. Horimatsu T, Patel AS, Prasad R, Reid LE, Benson TW, Zarzour A, Ogbi M, Bruder do Nascimento T, Belin de Chantemele E, et al. Remote effects of transplanted perivascular adipose tissue on endothelial function and atherosclerosis. Cardiovasc Drugs Ther. 2018;32:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Öhman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, De Meyer GRY. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. [DOI] [PubMed] [Google Scholar]
- 11. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov‐Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 12. Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, Nishio C, Higashida M, Nakaya Y, Kitagawa T, Sata M. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Hear J. 2011;52:139–142. [DOI] [PubMed] [Google Scholar]
- 13. Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, Higashida M, Mikasa H, Nakaya Y, Takanashi S, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58:248–255. [DOI] [PubMed] [Google Scholar]
- 14. Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, Ohba K, Matsubara J, Sumida H, Nagayoshi Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213:649–655. [DOI] [PubMed] [Google Scholar]
- 15. Tavora F, Kutys R, Li L, Ripple M, Fowler D, Burke A. Adventitial lymphocytic inflammation in human coronary arteries with intimal atherosclerosis. Cardiovasc Pathol. 2010;19:e61–e68. [DOI] [PubMed] [Google Scholar]
- 16. Verhagen SN, Vink A, van der Graaf Y, Visseren FLJ. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition: a post‐mortem study. Atherosclerosis. 2012;225:99–104. [DOI] [PubMed] [Google Scholar]
- 17. Kralova Lesna I, Tonar Z, Malek I, Maluskova J, Nedorost L, Pirk J, Pitha J, Lanska V, Poledne R. Is the amount of coronary perivascular fat related to atherosclerosis? Physiol Res. 2015;64(suppl 3):S435–S443. [DOI] [PubMed] [Google Scholar]
- 18. Mazurek T, Kobylecka M, Zielenkiewicz M, Kurek A, Kochman J, Filipiak KJ, Mazurek K, Huczek Z, Królicki L, Opolski G. PET/CT evaluation of (18)F‐FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: independent predictor of atherosclerotic lesions’ formation? J Nucl Cardiol. 2016;24:1075–1084. [DOI] [PubMed] [Google Scholar]
- 19. Mazurek T, Kochman J, Kobylecka M, Wilimski R, Filipiak KJ, Królicki L, Opolski G. Inflammatory activity of pericoronary adipose tissue may affect plaque composition in patients with acute coronary syndrome without persistent ST‐segment elevation: preliminary results. Kardiol Pol. 2014;72:410–416. [DOI] [PubMed] [Google Scholar]
- 20. Chistiakov DA, Orekhov AN, Bobryshev YV. Immune‐inflammatory responses in atherosclerosis: role of an adaptive immunity mainly driven by T and B cells. Immunobiology. 2016;221:1014–1033. [DOI] [PubMed] [Google Scholar]
- 21. Tay C, Liu Y‐H, Hosseini H, Kanellakis P, Cao A, Peter K, Tipping P, Bobik A, Toh B‐H, Kyaw T. B‐cell‐specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc Res. 2016;111:385–397. [DOI] [PubMed] [Google Scholar]
- 22. Ketelhuth DFJ, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res. 2016;118:668–678. [DOI] [PubMed] [Google Scholar]
- 23. Salonen JT, Korpela H, Salonen R, Nyyssonen K, Yla‐Herttuala S, Yamamoto R, Butler S, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. [DOI] [PubMed] [Google Scholar]
- 24. Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat‐shock protein 70 and signaling through toll‐like receptor‐4. Circulation. 2002;105:685–690. [DOI] [PubMed] [Google Scholar]
- 26. Farias‐Itao DS, Pasqualucci CA, Nishizawa A, Silva LFF, Campos FM, da Silva KCS, Leite REP, Grinberg LT, Ferretti‐Rebustini REL, Jacob Filho W, Suemoto CK. Perivascular adipose tissue inflammation and coronary artery disease: an autopsy study protocol. JMIR Res Protoc. 2016;5:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lucas S. The autopsy pathology of sepsis‐related death. Curr Diagnostic Pathol. 2007;13:375–388. [Google Scholar]
- 28. Tsokos M. Postmortem diagnosis of sepsis. Forensic Sci Int. 2007;165:155–164. [DOI] [PubMed] [Google Scholar]
- 29. Stary H. Natural history and histological classification of atherosclerotic lesions: an update. Arter Thromb Vasc Biol. 2000;20:1177–1178. [DOI] [PubMed] [Google Scholar]
- 30. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. [DOI] [PubMed] [Google Scholar]
- 31. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suemoto CK, Nitrini R, Grinberg LT, Ferretti RE, Farfel JM, Leite RE, Menezes PR, Fregni F, Jacob‐Filho W, Pasqualucci CA; Brazilian Aging Brain Study Group . Atherosclerosis and dementia: a cross‐sectional study with pathological analysis of the carotid arteries. Stroke. 2011;42:3614–3615. [DOI] [PubMed] [Google Scholar]
- 33. Re Ferretti, Damin A, Bruck S, Morillo L, Perroco T, Campora F, Moreira E, Balbino É, Lima M, Battela C, Ruiz L, et al. Post‐mortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010;4:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. UCLA: Institute for Digital Research & Education . Regression with Stata chapter 4 – beyond OLS [Internet]. https://stats.idre.ucla.edu/stata/webbooks/reg/chapter4/regressionwith-statachapter-4-beyond-ols-2/. Accessed October 24, 2019.
- 35. Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, Wan W, Tai X. An update on inflamm‐aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bailey‐Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity‐induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti‐inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 38. Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. [DOI] [PubMed] [Google Scholar]
- 39. Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. [DOI] [PubMed] [Google Scholar]
- 40. Gu H, Gao Y, Wang H, Hou Z, Han L, Wang X, Lu B. Sex differences in coronary atherosclerosis progression and major adverse cardiac events in patients with suspected coronary artery disease. J Cardiovasc Comput Tomogr. 2017;11:367–372. [DOI] [PubMed] [Google Scholar]
- 41. Kosyreva AM, Makarova OV, Kakturskiy LV, Mikhailova LP, Boltovskaya MN, Rogov KA. Sex differences of inflammation in target organs, induced intraperitoneal injection of lipopolysaccharide, depend on its dose. J Inflamm Res. 2018;11:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546. [DOI] [PubMed] [Google Scholar]
- 43. Singh P, Castillo A, Islam MT, Majid DSA. Evidence for prohypertensive, proinflammatory effect of interleukin‐10 during chronic high salt intake in the condition of elevated angiotensin ii level novelty and significance. Hypertension. 2017;70:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruan C‐C, Ma Y, Ge Q, Li Y, Zhu L‐M, Zhang Y, Kong L‐R, Wu Q‐H, Li F, Cheng L, Zhao AZ, Zhu D‐L, Gao P‐J. Complement‐mediated inhibition of adiponectin regulates perivascular inflammation and vascular injury in hypertension. FASEB J. 2017;31:1120–1129. [DOI] [PubMed] [Google Scholar]
- 45. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 46. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 47. Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689–702. [DOI] [PubMed] [Google Scholar]
- 48. Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson PWF. Established risk factors and coronary artery disease: the framingham study. Am J Hypertens. 1994;7:7S–12S. [DOI] [PubMed] [Google Scholar]
- 50. Hojbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, Bruun JM, Stallknecht B. Impact of physical inactivity on adipose tissue low‐grade inflammation in first‐degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34:2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clément K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri‐atrial, peri‐ventricular, or peri‐coronary location. Cardiovasc Res. 2015;108:62–73. [DOI] [PubMed] [Google Scholar]
- 52. Stöger JL, Gijbels MJJ, van der Velden S, Manca M, van der Loos CM, Biessen EAL, Daemen MJAP, Lutgens E, de Winther MPJ. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. [DOI] [PubMed] [Google Scholar]
- 53. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rehg JE, Bush D, Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol. 2012;40:345–374. [DOI] [PubMed] [Google Scholar]
- 55. Ait‐Oufella H, Herbin O, Bouaziz J‐D, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, et al. Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;85:4410–4419. [DOI] [PubMed] [Google Scholar]
- 57. Zouggari Y, Ait‐Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig‐Martin SA, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high‐fat feeding. Circ Res. 2009;104:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Skiba DS, Nosalski R, Mikolajczyk TP, Siedlinski M, Rios FJ, Montezano AC, Jawien J, Olszanecki R, Korbut R, Czesnikiewicz‐Guzik M, et al. Anti‐atherosclerotic effect of the angiotensin 1‐7 mimetic AVE0991 is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis. Br J Pharmacol. 2017;174:4055–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uchida Y, Uchida Y, Shimoyama E, Hiruta N, Kishimoto T, Watanabe S, Szasz T, Webb C, Lee DE, Kehlenbrink S, et al. Human pericoronary adipose tissue as storage and possible supply site for oxidized low‐density lipoprotein and high‐density lipoprotein in coronary artery. J Cardiol. 2016;69:1–12. [DOI] [PubMed] [Google Scholar]
- 61. Fernández‐Alfonso MS, Gil‐Ortega M, Aranguez I, Souza D, Dreifaldt M, Somoza B, Dashwood MR. Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol. 2017;174:3561–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gomez‐Nicola D, Boche D. Post‐mortem analysis of neuroinflammatory changes in human Alzheimer's disease. Alzheimers Res Ther. 2015;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suleiman M‐S, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open‐heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suemoto CK, Ferretti‐Rebustini RE de L, Rodriguez RD, Leite REP, Soterio L, Brucki SMD, Spera RR, Cippiciani TM, Farfel JM, Chiavegatto‐Filho A, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross‐sectional study. PLoS Med. 2017;14:e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Farfel JM, Nitrini R, Suemoto CK, Grinberg LT, Ferretti REL, Leite REP, Tampellini E, Lima L, Farias DS, Neves RC, et al. Brazilian Aging Brain Study Group . Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology. 2013;81:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptive Analysis of Coronary Artery Atherosclerosis and Immune Cells in the Perivascular Adipose Tissue
Table S2. Association of Density of CD68+ Macrophages in the PvAT With Sociodemographic and Clinical Data (n=246)
Table S3. Association of Density of CD3+ T‐Lymphocytes in the PvAT with Sociodemographic and Clinical Data (n=246)
Table S4. Association of Density of CD20+ B‐Lymphocytes in the PvAT with Sociodemographic and Clinical Data (n=246)
Table S5. Comparison of Immune Cells Between Perivascular Adipose Tissue of Unstable Atherosclerosis and Perivascular Adipose Tissue of Stable Atherosclerosis in the Same Individual (n=26 matched pairs)
Figure S1. Signs of sepsis detected during the anatomopathological post‐mortem exam. A, microscopic photograph (×10 magnification) of lung with neutrophil infiltration due to bronchopneumonia. B, microscopic photograph (×40 magnification) of neutrophil infiltrate in the lung. C, microscopic photograph (×40 magnification) acute tubular necrosis responsive to shock in the kidney; D, microscopic photograph (×10 magnification) of acute splenitis; E, microscopic photograph (×10 magnification) of hepatic congestion and steatosis secondary to bronchopneumonia.
Figure S2. Coronary artery with intraplaque hemorrhage. A, Coronary artery fragment stained with haematoxylin‐eosin (×2 of magnification). I: erythrocytes (×40 of magnification); II: lipid core (×40 of magnification); III: narrowing of the fibrous cap adjacent to the intraplaque hemorrhage (×40 ofmagnification). B, Coronary artery fragment stained with Masson's trichrome (×2 ofmagnification). I: erythrocytes (×40 of magnification); II: lipid core (×40 of magnification); III: narrowing of fibrous cap adjacent to intraplaque hemorrhage (×40 of magnification); IV: lipid core and intraplaque hemorrhage (×10 of magnification)and V: fibroses (×10 of magnification).
Figure S3. Coronary artery with thrombus. A,Coronary artery fragment stained with haematoxylin‐eosin (×2 of magnification). I: lipidcore (×40 of magnification); II: erythrocytes inside the thrombus (×40 of magnification); III: calcification (×40 of magnification). B, Coronary artery fragment stained with Masson'strichrome (×2 of magnification). I: Periplaque perivascular adipose tissue near the adventitialayer (×10 of magnification); II: vasa vasorum (×10 of magnification); III: thrombus (×40 of magnification).
Figure S4. Flowchart of study participants. HIV indicates Human Immunodeficiency Virus; SPAS, São Paulo Autopsy Service.
Figure S5. Association between CD3+ T‐lymphocytes and atherosclerosis. PvATp, periplaque perivascular adipose tissue; PvATd, distal perivascular adipose tissue. A, Scatter plot of the density of CD3+ T‐lymphocytes in periplaque PvAT with the percentage of coronary artery obstruction. B, Box plot graph of the density of CD3+ T‐lymphocytes in periplaque PvAT by stable and unstable plaque groups. C, Scatter plot of the difference of the CD3+ T‐lymphocytes density in periplaque PvAT and distal PvAT with the percentage of coronary artery obstruction. D, Box plot graph of the difference density of CD3+ T‐lymphocytes between periplaque PvAT and distal PvAT by stable and unstable plaque groups. PvATd indicates distal perivascular adipose tissue; PvATp, periplaque perivascular adipose tissue.


