Figure 3.
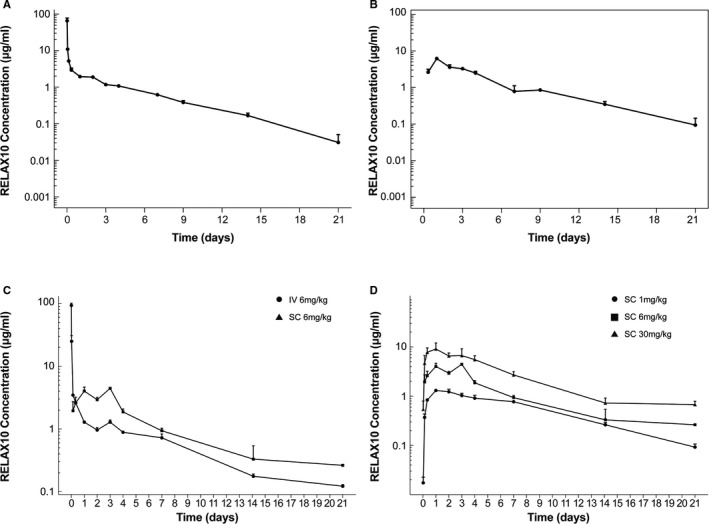
Pharmacokinetic profiles in rodent. The in vivo pharmacokinetic profiles of RELAX10 in rats was shown after intravenous (A) and subcutaneous (B) administration. The dose used for the rat pharmacokinetic study is 4 mg/kg, and blood sample collection occurs at various times after dosing. C, The pharmacokinetic profile of RELAX10 was also evaluated in mice after administration of a single 6 mg/kg dose by either IV or SC administration. D, A subcutaneous administration dose escalation pharmacokinetic study in mice to understand the relationship between the dose level and exposure. All the samples were tested by ELISA, in which an anti‐Fc monoclonal antibody TM446 (AstraZeneca) was used to coat the ELISA plate. The horseradish peroxidase (HRP)–conjugated polyclonal antibody from the Relaxin‐2 detection ELISA kit (R&D Systems) was used as the ELISA detection reagent.
