Figure 2.
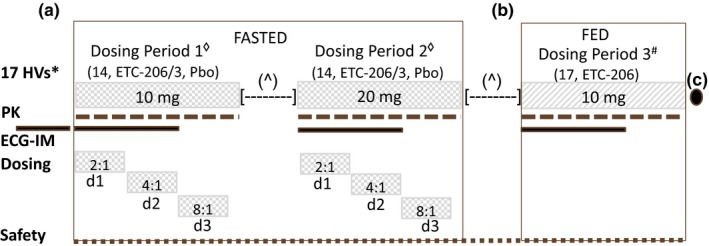
Study design outline, treatments, and key features. (a) Seventeen healthy volunteers were planned to receive single doses of ETC‐206 (n = 14) or placebo (n = 3) under fasted conditions in two consecutive dosing periods (DPs; 10 mg in DP1 and 20 mg in DP2). In each of the two DPs in fasted condition, ETC‐206 and Pbo were given in a staggered manner on day 1, day 2, and day 3 according to the ratio of 2:1, 4:1, 8:1, respectively. (b) In a subsequent DP3#, the entire group of 17 HVs was planned to receive a single dose of 10 mg of ETC‐206 (without Pbo) under fed condition. A minimum of 26 days [—] elapsed between doses for each subject. The next dose was administered after review (^) of safety, tolerability, and pharmacokinetic data from all subjects who received ETC‐206 or Pbo. Subjects were housed for 3 days and monitored ambulatory until day 7 postdose, during each DP. (c) A follow‐up visit was planned on day 14 (3), after the administration of ETC‐206 in the last DP. Electrocardiogram intensive monitoring – standard and triplicate (within 5 minutes) 12‐lead ECGs were performed prior to dosing and at 1, 2, 8, and 24 hours (±10 minutes) after dosing, with the triplicate ECG also being performed at 3, 6, 12, 36, and 48 hours (±10 minutes) after dosing. A 24‐hour continuous ECG recording was conducted on day −1 before DP1, and a 48‐hour continuous ECG recording was conducted on day 1 of each DP, starting prior to drug administration. d1, day 1; d2, day 2; d3, day 3; ECG‐IM, electrocardiogram intensive monitoring; HVs, healthy volunteers; Pbo, placebo; PK, pharmacokinetic.
