Abstract
The aim of this study was to investigate antibacterial activity of Origanum compactum essential oils collected at three phenological stages on Escherichia coli and Bacillus subtilis. The antibacterial activity was evaluated using the agar-well diffusion assay. The MIC and MBC values were determined using the micro-dilution assay. The investigation of the antibacterial action was carried out by the evaluation of the effect of O. compactum essential oils on the antibacterial kinetic growth, the integrity of cell membrane and permeability of the cell membrane. The anti-quorum sensing activity was tested by the inhibition of the biofilm formation. The findings of this study showed that O. compactum essential oil has potent antibacterial activities against E. coli and B. subtilis. The lowest inhibition value against B. subtilis was obtained with O. compactum essential oil at the post-flowering stage (MIC = MBC = 0.0312% (v/v)). The antibacterial mechanisms of O. compactum essential oils are related to the disturbing of the cell membrane integrity and the increasing of the membrane permeability, which leads to the leakage of genetic materials (DNA and RNA). Moreover, O. compactum essential oils inhibited the formation of the biofilms, a phenotype that has been known to be quorum sensing regulated.
Keywords: Origanum compactum, Essential oil, Antibacterial action, Cell membrane, Quorum sensing inhibition
1. Introduction
The emergence and re-emergence of infectious diseases are primarily caused by the ability of bacteria to withstand the antibiotics. Indeed, bacteria have developed sophisticated means to counteract the actions of antibiotics [1]. The implication of the quorum sensing in the establishment of resistance mechanisms by linking the interactions between bacteria is a recently deciphered mode of regulation. In addition, bacteria are the main cause of food deterioration in the food industry. The deterioration of food causes damage to the health of consumers and/or industry. Moreover, due to the toxicological side effects of synthetic antibacterial products and the problems associated with their biodegradation, efforts have been made recently to find effective alternatives to the pharmaceutical and food industries [2].
Essential oils are natural molecules of complex mixture that possess several chemical structures synthesized by aromatic plants as secondary metabolites [1]. The role of these essential oil molecules in plants is mainly related to the process of defense against pathogenic microorganisms. Essential oils have been found to have a variety of applications, particularly in the cosmetic and food industries and as a source of drugs [2,3]. In vitro and in vivo studies have shown that essential oils and their active compounds possess several biological effects such as antimicrobial, antioxidant, antitumor and anti-inflammatory properties [[2], [3], [4], [5]].
Origanum compactum Benth. (O. compactum) is an aromatic and medicinal plant known in Morocco by its vernacular names “Zaatar” and “Sahtar”. This species has long been used in the Moroccan pharmacopeia for its multiple medicinal virtues. Indeed, the aerial parts of O. compactum are used by the Moroccan population to fight against pulmonary and gastrointestinal infections [6,7]. Moreover, leaves and stems are used against the pathologies of the digestive system, heart diseases, inflammation, hypertension and diabetes [[8], [9], [10]]. Several studies have shown that O. compactum essential oils are rich in terpenic compounds of aromatic classes such as carvacrol, thymol, γ-terpinene and p-cymene [[11], [12], [13]]. However, this composition is much more affected by genetic determinism, environmental factors [14] and phenological stages of the plant [15].
In vitro studies showed the biological activities of O. compactum essential oils, particularly the antibacterial activity [11,12]. The antibacterial action of O. compactum essential oils was carried out by Bouhdid et al. [16] in a previous work against Pseudomonas aeruginosa and Staphylococcus aureus. In this study, O. compactum essential oils were altered at physiological and structural levels, which led to the death of bacterial cells [16]. However, the study could not correlate action targets and active compounds of O. compactum essential oils. Certainly, the mechanism of antibacterial action of the essential oils and their derivatives is attributed to the ability of the phenolic compounds to cross the cell membrane and consequently disturb its integrity. However, they can also deregulate the communication system between bacteria, thus causing them to lose their ability to coordinate the interactions between themselves and their environment in order to survive [17].
In a previous study, we evaluated the antibacterial, antileishmanial and antioxidant activities of O. compactum essential oils collected from the province of Ouezzane (North-West of Morocco) at the three developmental stages [15]. The results obtained were remarkably encouraging, particularly with regard to the antibacterial activity. Indeed, O. compactum essential oils tested at three phenological stages showed bacteriostatic and bactericidal effects at very low concentrations, especially against E. coli and B. subtilis. In order to understand the mechanisms leading to the death of these two bacteria, O. compactum essential oils collected at different phenological stages were tested. We also examined the action of O. compactum essential oils on dynamic kinetics, membrane permeability, membrane integrity and quorum sensing suppression phenotype of B. subtilis and E. coli.
2. Materials and methods
2.1. Plant material and essential oils extraction
The aerial parts of O. compactum used in this study were collected at three phenological stages (vegetative stage (VS), flowering stage (FS) and post-flowering stage (PFS)) from the North-West of Morocco. O. compactum essential oils were extracted by hydrodistillation method using Clevenger-type apparatus. The obtained oils were dried by anhydrous sodium sulfate, weighed and stored at 4 °C until use. The chemical composition of O. compactum essential oils was determined in our previous study using GC-MS analysis [15].
2.2. Bacterial strains and growth conditions
Escherichia coli K12 (Laboratory of Food Microbiology, UCL, Belgium: MBLA) and Bacillus subtilis 6633 (German Collection of Microorganisms: DSM) were chosen to evaluate the antibacterial activity of O. compactum essential oils. Strains were maintained on an inclined agar medium at 4 °C. Before use, the bacteria were revived by two subcultures in an appropriate culture medium: Lysogeny broth (LB) (Biokar Diagnostics, Beauvais, France) at 37 °C for 18–24 h. For the test, final inoculums concentrations of 106 CFU/mL bacteria were used according to the National Committee for Clinical Laboratory Standards, USA (NCCLS 1999).
2.3. Analysis of antibacterial activity
2.3.1. Agar-well diffusion assay
In order to estimate the bacteriostatic activity of O. compactum essential oils, the growth inhibition zone of germs around wells was measured. 6 mL of LB medium in superfusion containing 0.8% agar was inoculated by a fresh culture of indicator bacterial strain (a final concentration was 106 CFU/mL). After solidification, the wells were filled with 50 μL of O. compactum essential oils. After incubation at the appropriate temperature for 24 h, all plates were examined for any zone of growth inhibition, and the diameter of these zones was measured in millimeters [15]. All the tests were performed in triplicates.
2.3.2. Minimal inhibitory concentration (MIC)
MICs were determined using the broth micro-dilution assay as described by Bouyahya et al. [15]. Agar at 0.15% (w/v) was used as a stabilizer of the extract-water mixture and resazurin as a bacterial growth indicator. 50 μL of bacteriological agar (0.15%, w/v) was distributed from the 2nd to the 8th well of a 96-well polypropylene microtitre plate. The dilutions of O. compactum essential oils were prepared in Mueller Hinton Broth supplemented with bacteriological agar (0.15%, w/v) to reach a final concentration of 2%; 100 μL of these suspensions were added to the first test well of each microtitre line, and then 50 μL of scalar dilution was transferred from the 2nd to the 8th well. The 8th well was considered as growth control because no essential oil was added. We then added 50 μL of a bacterial suspension to each well at a final concentration of approximately 106 CFU/mL. The final concentration of the essential oil was between 1% and 0.015% (v/v). Plates were incubated at 37 °C for 18 h. After incubation, 10 μL of resazurin was added to each well to assess bacterial growth. After further incubation at 37 °C for 2 h, the MIC was determined as the lowest essential oils concentration that prevented a change in resazurin color. Bacterial growth was detected by a reduction in blue dye resazurin to pink resorufin. A control was carried out to ensure that at the concentrations tested, the essential oils did not cause a color change in the resazurin. Experiments were performed in triplicates.
2.3.3. Determination of minimal bactericidal concentration (MBC)
MBC, corresponding to the lowest concentration of the essential oil yielding negative subcultures after incubation at the appropriate temperature for 24 h, was determined in broth dilution tests by sub-culturing 10 μL from negative wells on plate count agar medium. All the tests were performed in triplicates [15].
2.4. Mechanisms of antibacterial action
2.4.1. Antibacterial kinetics assay
The growth curve assay was used to investigate the bactericidal effects of the essential oil. Bacteria were treated with the essential oil at MIC, 2×MIC, MIC/2, and the control containing only DMSO (1%). Then the bacteria were cultivated at 37 °C for 0, 2, 4, 6, 8, 10, 12, and 24 h, respectively. At selected time intervals, the measure of the optical density of supernatants was determined by UV–Vis spectrophotometer. All measurements were carried out in triplicate. Through the assay above, the growth curve of E. coli and B. subtilis was ordered, the time as the horizontal axis (0 h–24 h), and the OD600 nm of the supernatant as the vertical axis [18].
2.4.2. Cell membrane integrity
2.4.2.1. Leakage of DNA and RNA through the bacterial membrane
The integrity of the cell membrane could be monitored by the release of cytoplasmic constituents of the cell [19]. The experiments were designed as follows: the bacteria were incubated at 37 °C for 12 h. Logarithmic growth phase cells of bacteria were treated with the essential oil at MIC, 2×MIC and MIC/2 value except for the control. Then the samples were incubated at 37 °C for 24 h. The samples were then immediately centrifuged (10,000 g) for 5 min at 4 °C. To determine the amounts of the DNA and RNA released from the cytoplasm, the supernatant was used to measure the optical density at 260 nm.
2.4.2.2. Leakage of proteins through the bacterial membrane
The cell integrity was examined by determining the release of proteins into the supernatant. The concentrations of proteins in supernatants were determined by the Bradford's method [20]. Logarithmic growth phase was estimated for bacteria cells treated with the essential oil at MIC, 2×MIC and MIC/2 except the control, during an incubation period of 24 h at 37 °C. Bacteria were separated by centrifugation (10,000 g) for 5 min at 4 °C, and the concentrations of cytoplasmic proteins were determined in the supernatant by measuring the optical density at 595 nm.
2.4.3. Permeability of cell membrane
The membrane permeability assay was monitored by neighboring nitrobenzene beta-d-galactose glucoside (ONPG) (Ryon) as described by Li et al. [21]. Briefly, bacteria suspension was diluted to 107 CFU/mL. Then ONPG (25 mmol/mL) and O. compactum essential oils were added to the suspension, the final concentrations of essential oils were MIC, 2×MIC and MIC/2. Physiological saline was used as a control. The inoculated suspension was incubated at 37 °C and samples were collected at 0, 1, 2, 4 and 8 h, respectively. The absorbance of sample supernatant was measured by spectrophotometer at 420 nm.
2.4.4. Anti-quorum sensing activity
Anti-quorum sensing activity of O. compactum essential oils was carried out by the measure of biofilm inhibition (the major phenotype of quorum sensing). The biofilm formation assay was performed in 96-well polystyrene plates as previously reported by several studies [22,23]. An overnight culture of E. coli and B. subtilis was diluted with Luria-Bertani broth and grown for another 4 h. After addition of different concentrations of O. compactum essential oils (MIC, 2×MIC, and MIC/2), 100 μL aliquots (107 CFU/mL) of culture were inoculated into the wells of the microtiter dishes and incubated for 24 h at 37 °C. Thereafter, we removed the medium and added 100 μL crystal violet 1% (w/v), dissolved in 95% ethanol, to each well. Following staining at room temperature for 20 min, the dye was removed and the wells were washed thoroughly with sterile water. For quantification of attached cells, the bound crystal violet was solubilized in dimethyl sulfoxide (DMSO) and the absorbance was determined at 570 nm to quantify total biofilms formation. The well without essential oils was considered as the negative control for the biofilm inhibition [24,25].
2.5. Statistical analysis
The statistical analysis was performed by a one-way analysis of variance (ANOVA). We considered that the difference is significant for p ≤ 0.05. The experiments were carried out for three replicates and the results are expressed as mean ± SD.
3. Results
3.1. Chemical composition
The chemical composition of essential oils of O. compactum at three phenological stages is summarized in Table 1. As listed, essential oils of O. compactum are rich oxygenated and hydrocarbons monoterpenes. Moreover, the main compounds of essential oils of O. compactum are carvacrol, thymol, p-cymene and γ-terpinene. However, the percentage yield of these main compounds changed from one stage of development to another. Indeed, the percentage of carvacrol and thymol (two main oxygenated monoterpenes) was 24.71% and 15.32% respectively at vegetative stage. Interestingly, a higher percentage of carvacrol (43.584%) than thymol (10.33%) was noted at the flowering stage. However, this percentage was reversed at the post-flowering stage (38.01% of thymol and 6.39% of carvacrol) (Table 1).
Table 1.
Chemical composition of essential oils of Origanum compactum aerial parts collected at three phenological stages [15].
| Compounds | Percentage of compounds |
||
|---|---|---|---|
| Vegetative | Flowering | Post-flowering | |
| α-Thujene | 1.35 | 0.87 | 0.65 |
| α -Pinene | 0.88 | 0.68 | 0.59 |
| 1-Octen-3-ol | 0.66 | 0.70 | 1.40 |
| 3-Octanone | 0.66 | 0.69 | 1.39 |
| β-Myrcene | 2.03 | 1.21 | 1.07 |
| α -Terpinene | 2.20 | 0.61 | 0.51 |
| P-Cymene | 17.81 | 18.58 | 19.24 |
| d-Limonene | 0.52 | 0.39 | 0.41 |
| 1,8-Cineole | 1.26 | 0.34 | 0.13 |
| γ-Terpinene | 17.41 | 8.71 | 7.11 |
| Linalool | 1.75 | 1.94 | 1.96 |
| Terpinen-4-ol | 0.78 | 0.78 | 1.10 |
| α-Terpineol | 2.89 | 3.25 | 7.35 |
| Thymol | 15.32 | 10.33 | 38.01 |
| Carvacrol | 24.71 | 43.58 | 6.39 |
| β-Caryophyllene | 1.90 | 0.60 | 0.43 |
| Caryophyllene oxide | 1.07 | 2.29 | 2.82 |
3.2. Antibacterial effect, MIC and MBC
Essential oils of O. compactum at three phenological stages (vegetative, flowering and post-flowering) were tested for their antibacterial activity in our previous study [15]. The results showed an interesting antibacterial activity (Table 2). The diameter of inhibition values showed that the essential oils of O. compactum had a broader spectrum of antibacterial inhibition than those proved by chloramphenicol and streptomycin used as positive controls. The MIC and MBC values ranged between 0.5% and 0.031% (v/v). The essential oils of O. compactum at vegetative and flowering stages had the similar MIC and MBC values against E. coli and B. subtilis, whereas the essential oils of O. compactum at post-flowering stage showed the lowest MIC and MBC values.
Table 2.
Antibacterial activity of O. compactum essential oils at three phenological stages.
| Strains | Vegetative stage |
Flowering stage |
Post-flowering stage |
Erythromycin |
Chloramphenicol |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | MIC | MBC | Diameter (mm) | MIC | MBC | Diameter (mm) | MIC | MBC | Diameter (mm) | MIC | MBC | Diameter (mm) | MIC | MBC | |
| B. subtilis | 26 ± 0.90b | 0.5 | 0.5 | 29 ± 1.16b | 0.625 | 0.625 | 31 ± 0.86c | 0.031 | 0.031 | na | 8 | 16 | 17 ± 0.25a | 8 | 8 |
| E. coli | 19 ± 0.58a | 0.25 | 0.25 | 31 ± 0.91c | 0.062 | 0.062 | 26 ± 0.73b | 0.25 | 0.25 | 21 ± 2.66a | 16 | 32 | 33 ± 0.5c | 8 | 32 |
Values in the same row not sharing a common letter (a-b-c) differ significantly at p < 0.05.
Final bacterial density was around 106 CFU/mL.
Diameter of inhibition zone including well diameter of 6 mm, by the agar-well diffusion method at a concentration of 50 μL of oil/well.
MIC: Minimum inhibitory concentration (% (v/v)).
MBC: Minimum bactericidal concentration (% (v/v)).
3.3. Antibacterial mechanisms
3.3.1. Antibacterial kinetics of essential oils
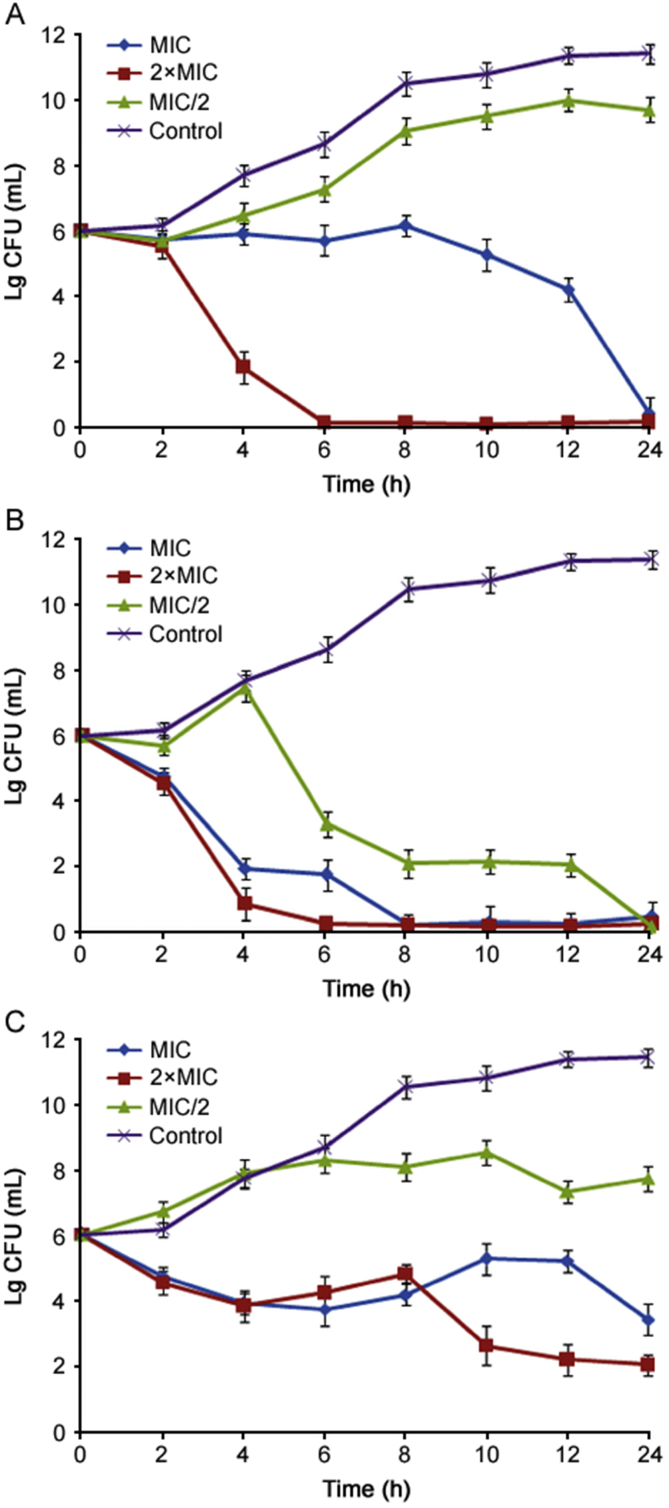
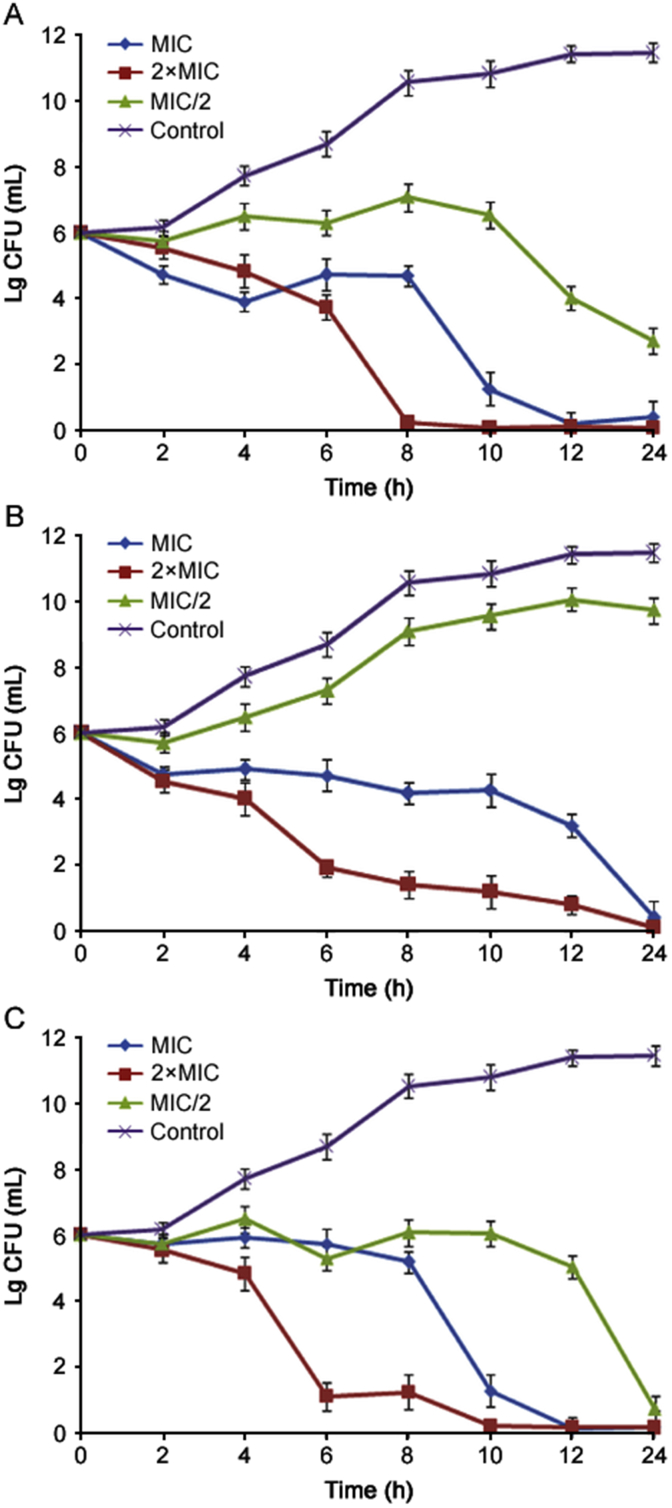
Essential oils of O. compactum were tested against the kinetics of bacterial growth of E. coli and B. subtilis. The growth curves of E. coli and B. subtilis affected by the essential oils of O. compactum are presented in Fig. 1, Fig. 2, respectively. As summarized, essential oils of O. compactum proved the reduction in the bacterial growth of E. coli and B. subtilis, with all used concentrations. For E. coli, essential oils of O. compactum at flowering stage (Fig. 1B) showed significant inhibition on kinetic growth of E. coli when treated with 2×MIC, MIC and MIC/2. This oil affects the growth of bacteria at different phases. For B. subtilis, the best inhibition of bacterial growth was obtained significantly with essential oils of O. compactum at post-flowering stage (Fig. 2C).
Fig. 1.
The growth curves of E. coli affected by the essential oil of O. compactum collected at the vegetative stage (A), the flowering stage (B) and the post-flowering stage (C). Data are expressed as mean ± SD (n = 3).
Fig. 2.
The growth curves of B. subtilis affected by the essential oil of O. compactum collected at the vegetative stage (A), the flowering stage (B) and the post-flowering stage (C). Data are expressed as mean ± SD (n = 3).
3.3.2. Cell membrane integrity
3.3.2.1. Leakage of DNA and RNA
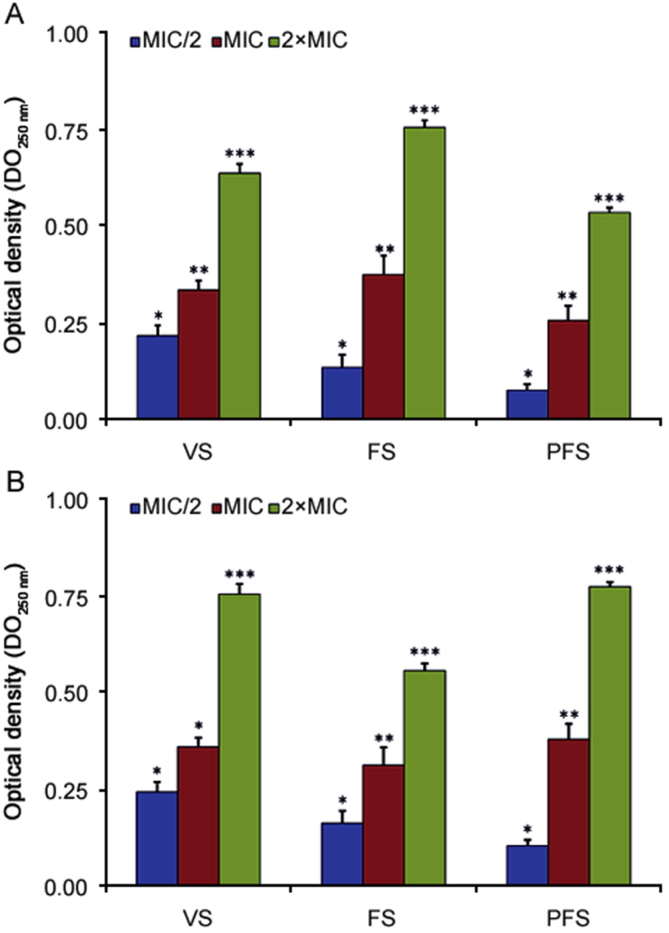
The leakage of the genetic materials through the bacterial membrane was used to reveal the action of O. compactum essential oils on the integrity of the membrane. The disruption of the membrane was determined by the measurement of cell constituents release (i.e. DNA and RNA) by assessing the absorbance at 260 nm in the supernatant of bacterial culture treated with essential oils of O. compactum at three phenological stages. The results, summarized in Fig. 3, indicated that for bacteria exposed to O. compactum essential oils at three phenological stages, the cell constituent's release (DNA and RNA) increased significantly in an essential oil concentration-dependent manner.
Fig. 3.
Release of genetic material (DNA and RNA) from E. coli (A) and B. subtilis (B) treated with the essential oils of O. compactum collected at three phenological stages: VS (vegetative stage), FS (flowering stage) and PFS (post-flowering stage) measured at 260 nm. The results are expressed as the absorbance of the sample (treated) – the absorbance of the control (no treated). Data are expressed as mean ± SD (n = 3). Values not sharing a common singe (*, **, ***) differ significantly at p < 0.05.
Essential oils of O. compactum at the flowering and vegetative stages induced important release of genetic materials from E. coli (Fig. 3A). The OD260 values of supernatant from E. coli treated with essential oils of O. compactum at MIC/2 concentration were 0.25 ± 0.02, 0.17 ± 0.01, and 0.11 ± 0.01 for vegetative, flowering and post-flowering stages, respectively. After the treatment by MIC concentration, the genetic material release is more interestingly. At a high concentration (2×MIC) essential oils of O. compactum induced important leakages of DNA and RNA from E. coli with OD values of 0.67 ± 0.02, 0.79 ± 0.03 and 0.57 ± 0.01, respectively, at the vegetative, flowering, and post-flowering stages. At the post-flowering and vegetative stages essential oils of O. compactum induced the best leakages of DNA and RNA from B. subtilis (Fig. 3B). The leakage of DNA and RNA from B. subtilis treated with various concentrations (2×MIC, MIC, and MIC/2) of essential oils of O. compactum at three phenological stages also showed an increase in genetic materials leakage in a concentration-dependent manner.
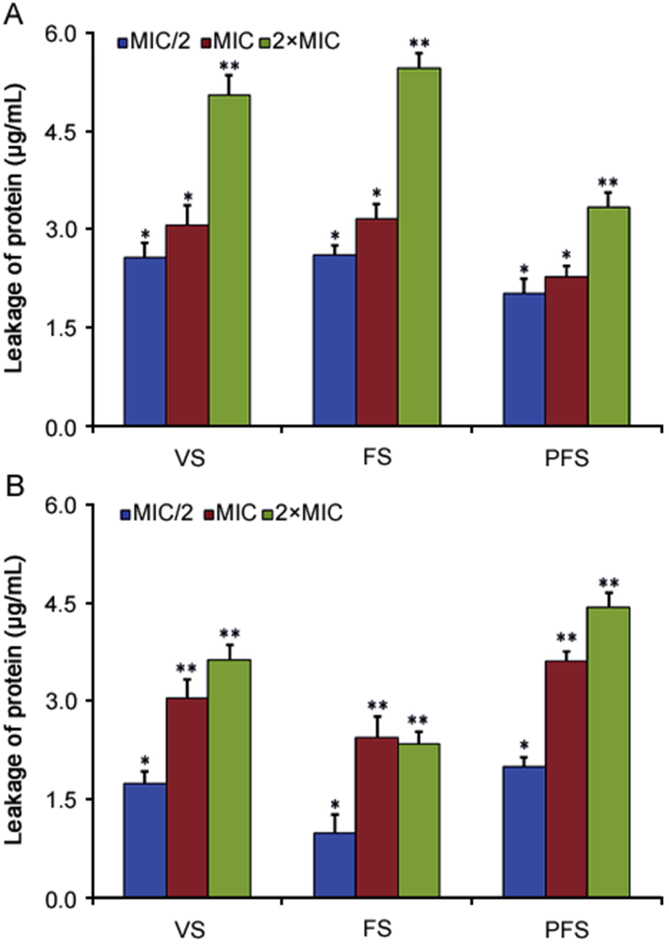
3.3.2.2. Leakage of proteins through the bacterial membrane
As shown in Fig. 4, essential oils of O. compactum at three phenological stages could enhance the leakage of proteins through the membrane of E. coli and B. subtilis. For E. coli, essential oils of O. compactum at flowering and vegetative stages were the most actives (Fig. 4A). Indeed, the leakage amount of proteins from E. coli treated with essential oils of O. compactum at the flowering stage was 5.47 ± 0.21 μg/mL (2×MIC), 3.16 ± 23 μg/mL (MIC) and 2.62 ± 0.14 μg/mL (MIC/2), respectively. In contrast the leakage amount of proteins from E. coli treated with O. compactum essential oils at the vegetative stage were 5.05 ± 0.3 μg/mL (2×MIC), 3.07 ± 0.29 μg/mL (MIC) and 2.57 ± 0.22 μg/mL (MIC/2), respectively. These leakage amounts of proteins from E. coli treated were higher than those (0.53 ± 0.2 μg/mL) from E. coli without treatment (control) (Fig. 4A).
Fig. 4.
Leakage of proteins from E. coli (A) and B. subtilis (B) treated with the essential oil of O. compactum collected at three phenological stages: VS (vegetative stage), FS (flowering stage) and PFS (post-flowering stage). The results are expressed as the absorbance of the sample (treated) – the absorbance of the control (no treated). Data are expressed as mean ± SD (n = 3). Values not sharing a common singe (*, **, ***) differ significantly at p < 0.05.
Therefore, the cells of B. subtilis importantly released proteins before its exposure to the essential oil of O. compactum at the post-flowering and vegetative stages (Fig. 4B). Indeed, the leakage amount of proteins from B. subtilis treated with O. compactum essential oils at the post-flowering stage was 5.25 ± 0.23 μg/mL (2×MIC), 4.39 ± 0.15 μg/mL (MIC) and 3.02 ± 0.14 μg/mL (MIC/2), respectively. In contrast, the leakage amount of proteins from B. subtilis treated with the essential oils of O. compactum at vegetative stage was 4.45 ± 0.22 μg/mL (2×MIC), 3.87 ± 0.28 μg/mL (MIC) and 2.56 ± 0.18 μg/mL (MIC/2), respectively.
These leakage amounts of proteins from B. subtilis treated were higher than that (0.83 ± 0.19 μg/mL) from B. subtilis without treatment (control). Moreover, the leakage of proteins from E. coli and B. subtilis cells either treated with essential oils of O. compactum increased in a concentration-dependent manner.
3.3.3. Permeability of cell membrane
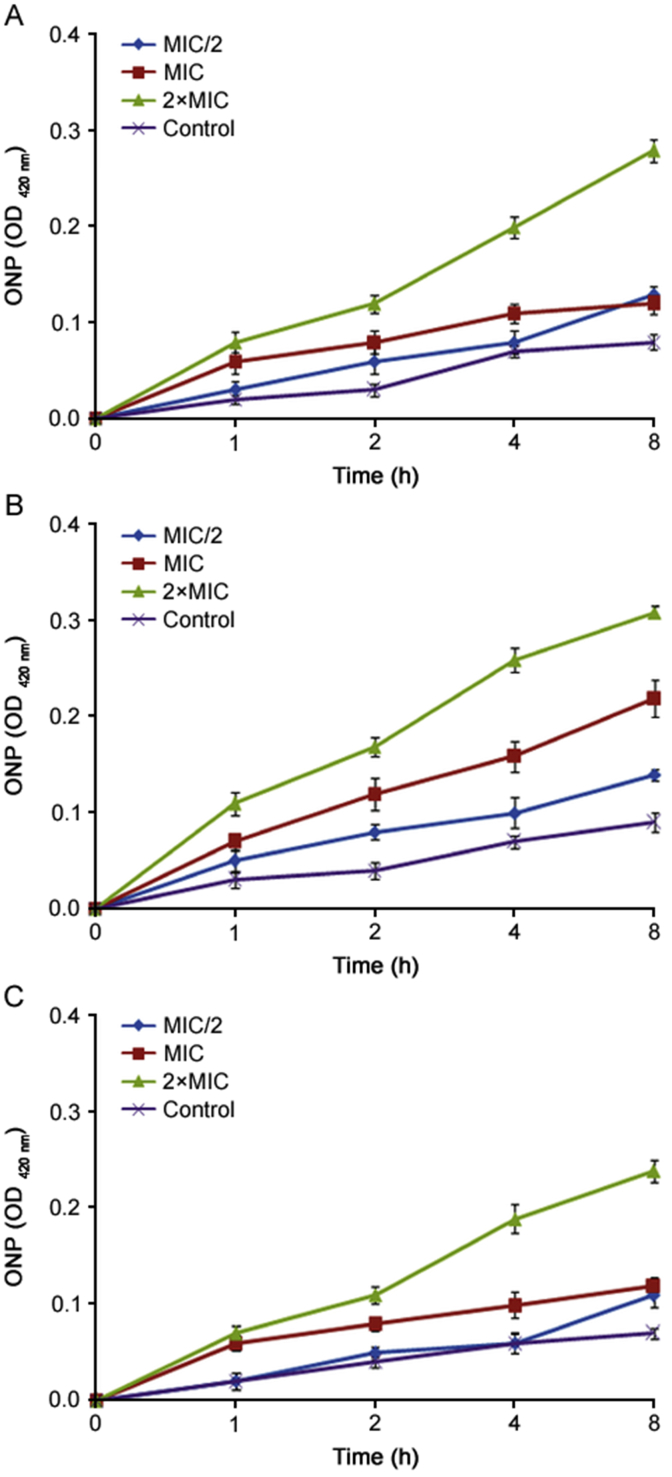
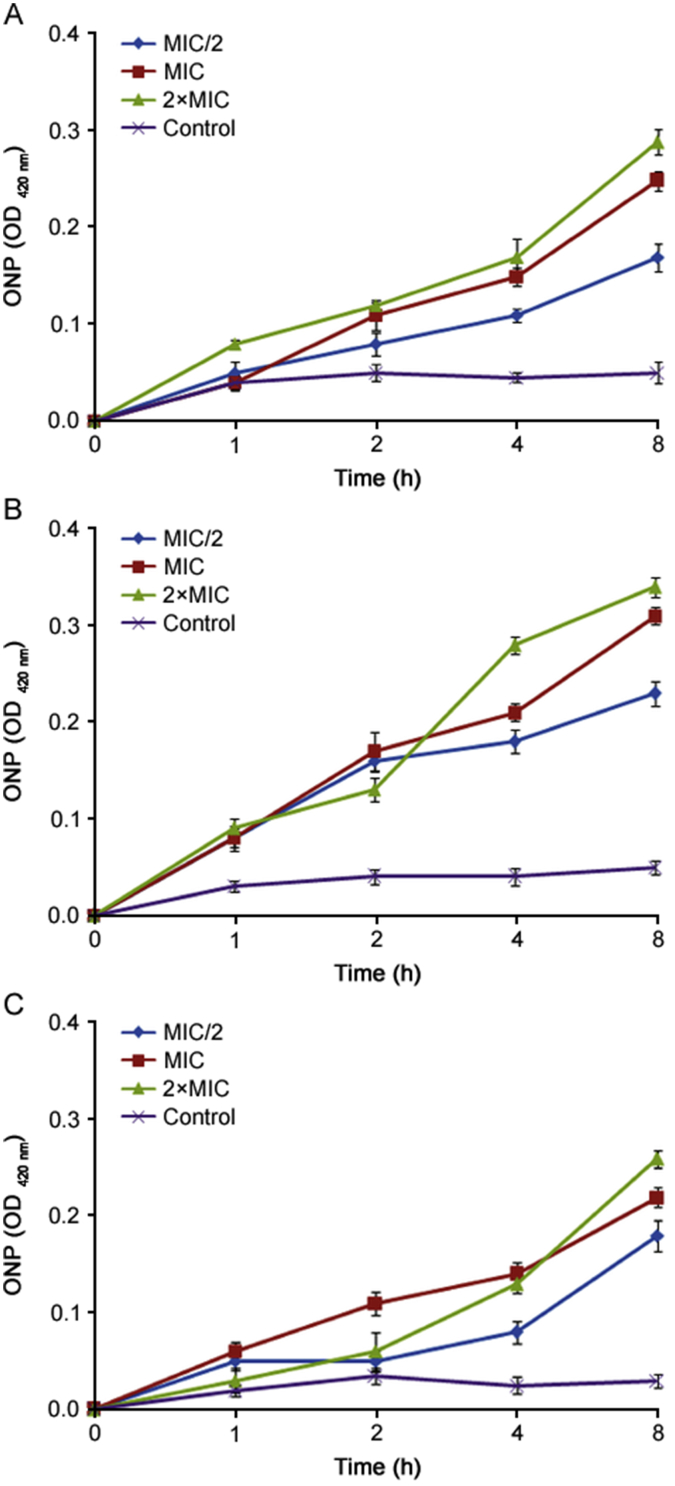
Further antibacterial mode of action of the essential oils of O. compactum at three phenological stages against E. coli and B. subtilis was confirmed using the assay of cell membrane permeability. The membrane permeability of bacteria was measured by the ONP subtract. The obtained results of the effects of essential oils of O. compactum against membrane permeability are summarized in Fig. 5 and Fig. 6. As indicated, the results showed that when treated with essential oils of O. compactum at 2×MIC and MIC, absorbance values of ONP in treated bacteria increased directly and they also increased with increased time of exposure and concentration of essential oils of O. compactum. It means that the permeability of E. coli and B. subtilis membrane would be increased, which caused the leakage of intracellular ingredients.
Fig. 5.
The OD420 nm values of ONP measured after the action of the essential oil of O. compactum collected at the vegetative stage (A), the flowering stage (B) and the post-flowering stage (C) against E. coli. Data are expressed as mean ± SD (n = 3).
Fig. 6.
The OD420 nm values of ONP measured after the action of the essential oil of O. compactum collected at the vegetative stage (A), the flowering stage (B) and the post-flowering stage (C) against B. subtilis. Data are expressed as mean ± SD (n = 3).
3.3.4. Anti-quorum sensing activity
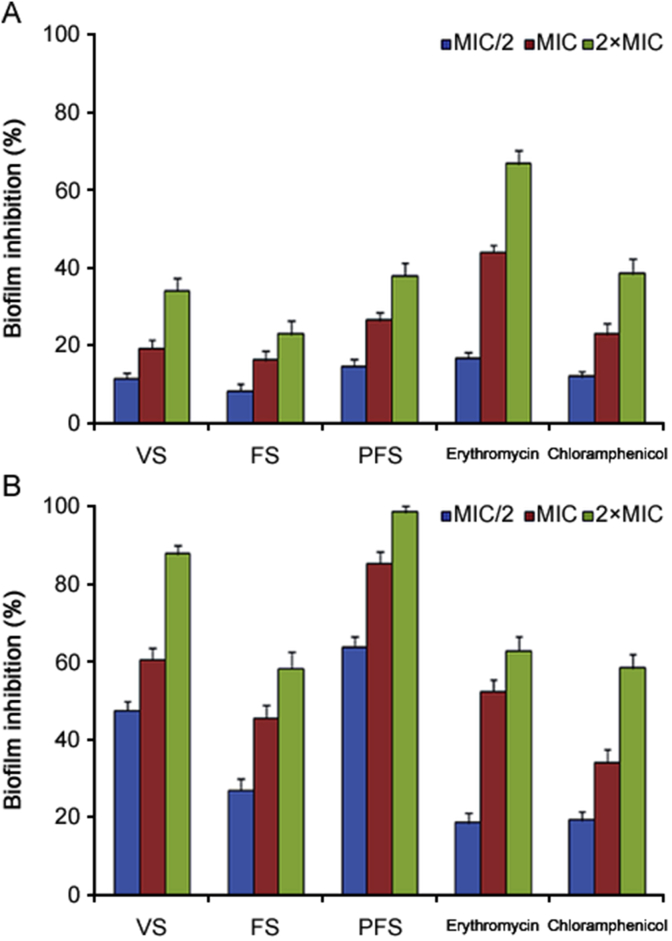
The anti-quorum sensing effects of O. compactum essential oils against E. coli and B. subtilis were measured by evaluating their capacity to inhibit the initial bacteria cell attachment (biofilm inhibition). Results of inhibition of bacteria attachment by O. compactum essential oils at three concentrations (2×MIC, MIC and MIC/2) are presented in Fig. 7. As summarized, the essential oil of O. compactum at the vegetative and post-flowering stages seemed to inhibit (98.32% and 87.48% inhibition activity respectively) bacteria cell attachment of B. subtilis at 2×MIC, while the percentage of biofilm formation by the essential oil of O. compactum at the flowering stage was moderate (Fig. 7B). E. coli was more resistant than B. subtilis as observed and proved by lower percentage inhibition values. The inhibition percentages of biofilm formation for E. coli were obtained at 2×MIC concentration of the essential oil of O. compactum at the vegetative (34.06%) and post-flowering stages (37.94%) (Fig. 7A).
Fig. 7.
Inhibition of biofilm formation in E. coli (A) and B. subtilis (B) by the essential oils of O. compactum collected at three phenological stages and control (erythromycin and chloramphenicol). Data are expressed as mean ± SD (n = 3).
4. Discussion
Bacteria have a particular growth period characterized by an important phase: exponential phase. The time required to reach this phase depends on the micro-organism and culture medium [26,27]. To reveal the ability of essential oils of O. compactum to lower the growth rate at the exponential level, we studied their action at different time points in order to show the rate of growth reduction.
The study of bacterial inhibition through growth kinetics is a very useful indication of the level of antibacterial action of the tested product. This gives important suggestions on the bacterial recalcitrance with respect to a product according to its phase of growth. Indeed, the action of antibacterial agents should be evaluated on the phase of exponential growth, phase for which the bacteria have a maximum growth rate, thus revealing the in vivo life state of the bacterium under the conditions of pathology.
In our work, essential oils showed significant effects on the kinetics of bacterial growth of B. subtilis and E. coli. Indeed, at sub-inhibitory concentrations (2×MIC), O. compactum essential oils inhibited the total growth (bactericidal action) of B. subtilis and E. coli. This result thus confirmed the bactericidal effect at MIC values. This action was recorded even in the exponential phase, which demonstrates the high bactericidal power of these tested oils. The decrease in cell viability of the tested strains indicates growth blockage accompanied by cell lysis. Other studies have revealed an important inhibition of the growth kinetics of E. coli and B. subtilis by O. compactum essential oils [16].
The cell membrane of bacteria is a barrier of selective permeability which organizes exchanges between the intra and extracellular environment by providing an intracellular environment suitable for the various vital processes. Moreover, the loss of this phenotype is often identified as an indicator of cell death [28]. Moreover, the bacterial cytoplasmic membrane provides a permeability barrier to the access of small ions such as K+, Na+, and H+, which are essential to facilitate cell membrane functions, maintain enzyme activity and keep the normal metabolism [29]. The impermeability to small ions is sustained and managed by the structure and chemical compositions of its membrane. Maintenance of ion homeostasis is of great importance to maintain the energy status of the cell, as it is significant to energy relevant processes such as solute transport, control of metabolism, management of pressure and motility [30]. Hence, even relatively minor variations of the structure of membranes can adversely affect cell metabolism and result in bacteria death [31].
Several techniques are used to evaluate the membrane permeability [16]. In our study, we opted for the use of the β-d-galactose glucoside (ONPG) method. The penetration of this substrate indicated that bacteria lose their capacity to control molecule penetration. The results indicated that essential oils of O. compactum increased the membrane permeability of two tested strains, particularly for B. subtilis. The recalcitrance of gram-positive bacteria is related to the nature of the complex cell wall that prevents the penetration of antimicrobial agents [1]. Moreover, the loss of permeability is remarkably obtained with the essential oil of O. compactum at the flowering stage (rich in carvacrol) and vegetative stage (rich in carvacrol and thymol) compared with at post-flowering stage (very rich in thymol) [15]. This clearly shows the ability of carvacrol to cross the cell membrane, and the property that was long suggested by several works [[32], [33], [34]].
Generally, essential oils and their constituents are characterized by a high hydrophobicity, which enables them to partition in the lipids of the bacterial cell membrane and mitochondria, disturbing the structures and rendering them more permeable [35]. Leakage of ions and other cell contents can then occur [30,36]. These results may be attributed to the carvacrol and thymol. Indeed, other studies showed that the antimicrobial effect of thymol and carvacrol is attributed to the perturbation of the lipid fraction of the bacterial membrane, resulting in the increase of membrane permeability [37].
The integrity of the cytoplasmic membrane is a critical factor to bacteria growth. Researchers have reported that the evaluation of cell leakage markers including absorbance at 260 nm for nucleic acid and the determination of proteins are indicators of membrane integrity [38]. Nucleic acids and proteins provide major structural functions and they are also involved in the transfer of cellular information in bacteria [39].
The present study indicated that the essential oil of O. compactum at flowering stage (rich in carvacrol) could act on the cytoplasmic membrane of E. coli, thus affecting the integrity of cytoplasmic membrane, while the cell membrane of B. subtilis was strongly affected by the essential oil of O. compactum at post-flowering stage (rich in thymol). Therefore, essential oil of O. compactum at three phenological stages reduced the content of cellular DNA, RNA and proteins by permeating and disrupting cell membrane integrity.
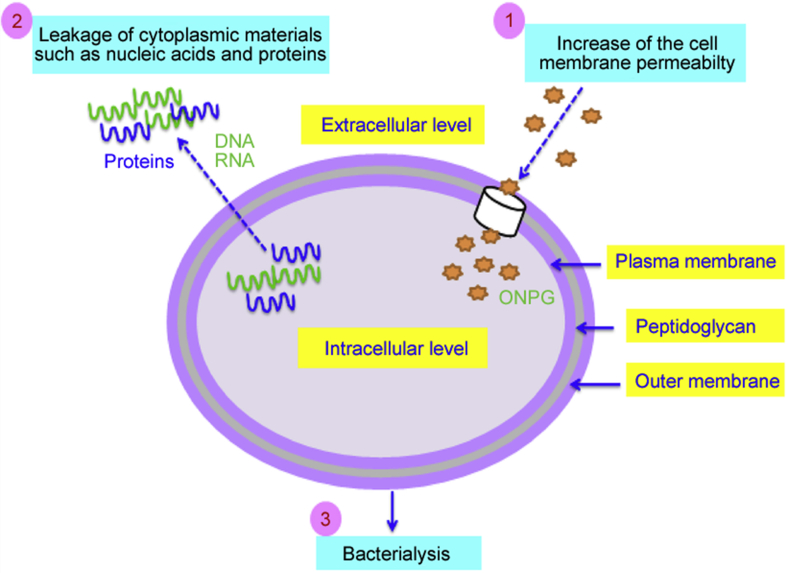
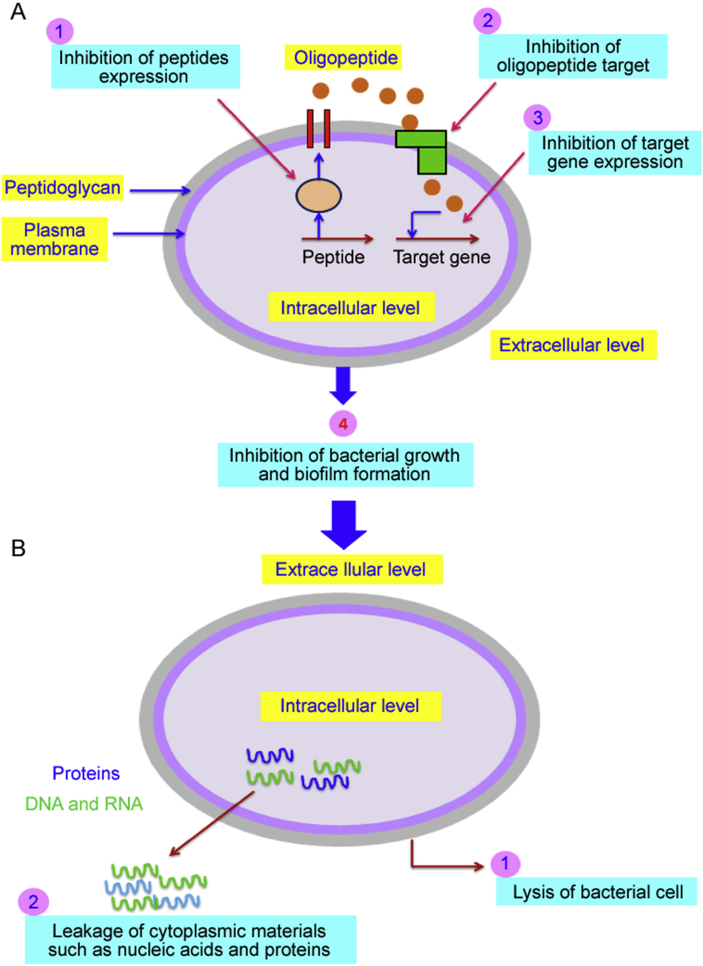
The results indicated that the irreversible damage to the cytoplasmic membranes might occur, which leads to the loss of cell constituents such as nucleic acid and proteins, and even cell death (Fig. 8). Moreover, our results are in concordance with other studies that have proved that the damage of cytoplasmic membranes of bacteria treated by essential oils leads to the loss of large molecules (DNA and RNA) and some proteins [29,40].
Fig. 8.
Possible antibacterial mechanisms of the essential oil of O. compactum against E. coli. The essential oils increased the membrane permeability that induced a disturbance in the membrane integrity justified by the release of cytoplasmic materials such as nucleic acids and proteins. In the end, the loss of membrane integrity led to cell lysis justified by the fall of bacterial growth dynamics.
However, it should be noted that essential oil of O. compactum at vegetative and flowering stages causes a greater loss of membrane integrity of E. coli, while the essential oil of O. compactum at post-flowering stage shows to be more bactericidal against B. subtilis. This result depends, certainly, on the major active compounds present in each essential oil. In a previous work, Xu et al. [32] showed that carvacrol induced a bactericidal action against E. coli. The mechanism associated with this action has been related to the perturbation of the potential of the membrane follows to a loss of its permeability. Other studies have also shown the antibacterial mechanisms of carvacrol against several bacteria including E. coli [41,42]. The carvacrol inhibited the efflux pump and down-regulated some gene expressions. On the other hand, the antibacterial action could be attributed to other molecules participating in the antibacterial effect [1].
In addition to their activities on the cell membrane, the essential oils may affect higher levels of regulation in bacteria. Indeed, bacteria coordinate their pathogenesis by communicating with each other through a communication system called quorum sensing. This system triggers certain specific phenotypes such as the formation of biofilms [17]. We have thus studied the action of essential oils of O. compactum against the quorum sensing by inhibiting the biofilm formation using the violet crystal as an indicator. Indeed, the attachment of the bacteria to each other and to the neighboring surfaces requires the formation of the biofilms. Biofilm is, therefore, an associative network of a bacterial community whose expression and functioning are governed by the quorum sensing [17].
To reveal the action of O. compactum essential oils against quorum sensing, their ability to inhibit the biofilm formation was tested. The essential oils of O. compactum inhibited the biofilm formation of E. subtilis more importantly than those of E. coli. In addition, the essential oil of O. compactum at post-flowering stage (rich in thymol) showed a very important anti-biofilm action, in particular its almost total inhibition against B. subtilis with sub-inhibitory concentrations.
The inhibition of quorum sensing in B. subtilis indicates the presence of cells that have maintained the membrane integrity but have lost the ability to form colonies due to lack of communication between them. Therefore, B. subtilis underwent cell lysis after a minimal maintenance state, which led to lysis of the cells after a long period incubation (Fig. 9). This result suggests that thymol (the main compounds of the essential oils of O. compactum at the post-flowering stage) has an anti-biofilm action against B. subtilis. Indeed, Raein et al. [43] showed that thymol and carvacrol strongly inhibited the biofilm formation of bacilli. In addition, thymol demonstrated anti-biofilm effects related to the inhibition of quorum sensing of other bacteria such as Salmonella enteric [44,45].
Fig. 9.
Possible antibacterial mechanisms of the essential oil of O. compactum against B. subtilis. (A) The essential oil inhibited the bacterial growth of B. subtilis by the inhibition of some intercellular communication pathways (oligopeptide of quorum sensing) justified by the inhibition of biofilm formation. The inhibited cells are maintained in a state of minimum viability. During this period, the cells remain isolated without any ability to divide (inhibition of their growth). (B) The inhibition of B. subtilis growth led to its lysis after some time, which leaked cytoplasmic materials such as nucleic acids and proteins.
Recently, several studies have focused on anti-quorum sensing effects of essential oils [[46], [47], [48]]. Indeed, the quorum sensing regulates several functions, in particular, this implication in biofilm formation, resistance to antibiotics and pathogenicity. The inhibition of biofilm formation by essential oils is certainly attributed to bioactive molecules that have specific target on quorum sensing signaling pathways [17]. Thus, essential oils and their active compounds could inhibit signaling pathways of the quorum sensing that are implicated of biofilm formation [49,50].
5. Conclusion
The trend of using essential oils as natural antimicrobial agents is, therefore, gradually becoming an attractive approach in the field of drug discovery and food preservation. The current research has explored the use of O. compactum essential oils as a natural antibacterial agent and gave insights into its mode of action on E. coli and B. subtilis as representatives of Gram-positive and Gram-negative bacteria. The essential oils of O. compactum exhibited significant antibacterial activity against E. coli and B. subtilis. The mechanism of action against E. coli may be due to the irreversible damage caused by O. compactum essential oils on the cell wall and membrane, leading to the leakage of proteins and genetic materials (DNA and RNA). However, the mechanism of action of essential oils of O. compactum against B. subtilis seems to be related to the quorum sensing deregulation. However, because the essential oils of O. compactum have many kinds of chemical constituents, it seems impossible that there is only one antibacterial mechanism or that only one component is responsible for the antibacterial activity. Therefore, further research such as separating the new chemical compounds, in vivo antibacterial activity and transmission electron microscopy is necessary to fully investigate the antibacterial mechanism of O. compactum essential oils against E. coli and B. subtilis.
Acknowledgments
The authors would like to thank the “Centre National pour la Recherche Scientifique et Technique” (CNRST) and “Agence Nationale des Plantes Médicinales et Aromatiques ” (ANPMA) for their funding supports.
Conflicts of interest
The authors declare that there are no conflicts of interests.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Bouyahya A., Bakri Y., Et-Touys A. Resistance to antibiotics and mechanisms of action of essential oils against bacteria. Phytothérapie. 2017 [Google Scholar]
- 2.Lahlou M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013;4:17–31. [Google Scholar]
- 3.Bakkali F., Averbeck S., Averbeck D. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 4.Bouyahya A., Et-Touys A., Bakri Y. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017;111:41–49. doi: 10.1016/j.micpath.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Bouyahya A., Bakri Y., Khay E.O. Antibacterial, antioxidant and antitumor properties of Moroccan medicinal plants: a review. Asian Pac. J. Trop. Dis. 2017;7:57–64. [Google Scholar]
- 6.Merzouki A., Ed-derfoufi F., Molero Mesa J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco) Fitoterapia. 2000;71:278–307. doi: 10.1016/s0367-326x(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 7.Bouyahya A., Abrini J., Et-Touys A. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur. J. Integr. Med. 2017;13:9–25. [Google Scholar]
- 8.Hachi M., Benkhnigue O., Hachi T. Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco) Lazaroa. 2016;37:135–144. [Google Scholar]
- 9.Jamila F., Mostafa E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014;154:76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Orch H., Zidane L., Douira A. Ethnobotanical study of the plants used in the treatment of the digestive diseases by the riverine population of the forest of Izarène. Int. J. Recent. Sci. Res. 2017;8:15213–15219. [Google Scholar]
- 11.Bouhdid S., Skali S.N., Idaomar M. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008;7:1563–1570. [Google Scholar]
- 12.Ouedrhiri W., Balouiri M., Bouhdid S. Mixture design of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: optimization of their antibacterial effect. Ind. Crops Prod. 2016;89:1–9. [Google Scholar]
- 13.Aboukhalid K., Lamiri A., Agacka-Mołdoch M. Chemical polymorphism of Origanum compactum grown in all natural habitats in Morocco. Chem. Biodivers. 2016;13:1126–1139. doi: 10.1002/cbdv.201500511. [DOI] [PubMed] [Google Scholar]
- 14.Aboukhalid K., Al Faiz C., Douaik A. Influence of environmental factors on essential oil variability in Origanum compactum Benth. growing wild in Morocco. Chem. Biodivers. 2017;14 doi: 10.1002/cbdv.201700158. [DOI] [PubMed] [Google Scholar]
- 15.Bouyahya A., Dakka N., Talbaoui A. Correlation between phenological changes, chemical composition and biological activities of the essential oil from Moroccan endemic Oregano (Origanum compactum Benth) Ind. Crops Prod. 2017;108:729–737. [Google Scholar]
- 16.Bouhdid S., Abrini J., Zhiri A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009;106:1558–1568. doi: 10.1111/j.1365-2672.2008.04124.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouyahya A., Dakka N., Et-Touys A. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian. Pac. J. Trop. Med. 2017;10:729–743. doi: 10.1016/j.apjtm.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D., Wang D.M., Yang L.N. A modified and improved assay based on microbial test system (MTS) to evaluate antioxidant activity. Food. Anal. Method. 2016;4:895–904. [Google Scholar]
- 19.Chen C.Z., Cooper S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/s0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 20.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Li L., Song X., Yin Z. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016;187:139–145. doi: 10.1016/j.micres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Taganna J.C., Quanico J.P., Perono R.M.G. Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J. Ethnopharmacol. 2011;134:865–871. doi: 10.1016/j.jep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 23.O'Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.H., Park J.H., Cho H.S. Antibiofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling. 2013;29:491–499. doi: 10.1080/08927014.2013.788692. [DOI] [PubMed] [Google Scholar]
- 25.Bazargani M.M., Rohloff G. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156–164. [Google Scholar]
- 26.Rowe S.E., Conlon B.P., Keren L. Persisters: methods for isolation and identifying contributing factors-a review. Methods Mol. Biol. 2016;1333:17–28. doi: 10.1007/978-1-4939-2854-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Swinnen I.A., Bernaerts K., Dens E.J. Predictive modelling of the microbial lag phase: a review. Int. J. Food Microbiol. 2004;94:137–159. doi: 10.1016/j.ijfoodmicro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Simões E.R.B., Santos E.A., de Abreu M.C. Biomedical properties and potentiality of Lippia microphylla Cham. and its essential oils. J. Intecult. Ethnopharmacol. 2015;4:256–263. doi: 10.5455/jice.20150610104841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diao W.D., Hu O.P., Zhang H. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. [Google Scholar]
- 30.Cox S.D., Mann C.M., Markham J.L. Determining the antimicrobial actions of tea tree oil. Molecules. 2001;6:87–91. [Google Scholar]
- 31.Sharma A., Bajpai V.K., Baek K.H. Determination of antibacterial mode of action of Allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J. Food Saf. 2013;33:197–208. [Google Scholar]
- 32.Xu J., Zhou F., Ji B.P. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008;47:174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 33.Mooyottu S., Flock G., Venkitanarayanan K. Carvacrol reduces Clostridium difficile sporulation and spore outgrowth in vitro. J. Med. Microbiol. 2017;66:1229–1234. doi: 10.1099/jmm.0.000515. [DOI] [PubMed] [Google Scholar]
- 34.Vasconcelos S.E.C.B., Melo H.M., Cavalcante T.T.A. Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin- and vancomycin-resistant Staphylococcus aureus. BMC Complement Altern. Med. 2017;17:462. doi: 10.1186/s12906-017-1968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikkema J., de Bont J.A., Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 36.Ultee A., Bennik M.H., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokolik C.G., Ben-Shabat-Binyamini R., Gedanken A. Proteinaceous microspheres as a delivery system for carvacrol and thymol in antibacterial applications. Ultrason. Sonochem. 2018;41:288–296. doi: 10.1016/j.ultsonch.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Bajpai V.K., Sharma A., Baek K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. [Google Scholar]
- 39.Zhang Y.B., Liu X.Y., Wang Y.F. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. [Google Scholar]
- 40.Talbaoui A., Jamaly N., Aneb M. Chemical composition and antibacterial activity of essential oils from six Moroccan plants. J. Med. Plants Res. 2012;6:4593–4600. [Google Scholar]
- 41.Patil S.D., Sharma R., Srivastava S. Downregulation of yidC in Escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PLoS One. 2013;8:57370. doi: 10.1371/journal.pone.0057370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miladi H., Zmantar T., Chaabouni Y. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb. Pathog. 2016;99:95–100. doi: 10.1016/j.micpath.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Raei P., Pourlak T., Memar M.Y. Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cell. Mol. Biol. 2017;63:108–112. doi: 10.14715/cmb/2017.63.5.20. [DOI] [PubMed] [Google Scholar]
- 44.Miladi H., Zmantar T., Kouidhi B. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017;104:56–63. doi: 10.1016/j.micpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Nostro A., Scaffaro R., D'Arrigo M. Study on carvacrol and cinnamaldehyde polymeric films: mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012;96:1029–1038. doi: 10.1007/s00253-012-4091-3. [DOI] [PubMed] [Google Scholar]
- 46.Luís Â., Duarte A., Gominho J. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016;79:274–282. [Google Scholar]
- 47.Myszka K., Schmidt M.T., Majcher M. Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int. Biodeterior. Biodegrad. 2016;114:252–259. [Google Scholar]
- 48.Oliveira B.D., Rodrigues A.C., Cardoso B.M.I. Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind. Crops Prod. 2016;84:59–66. [Google Scholar]
- 49.Szabó M.A., Varga G.Z., Hohmann Schelz J. Inhibition of quorum-sensing signals by essential oils. Phytother Res. 2010;24:782–786. doi: 10.1002/ptr.3010. [DOI] [PubMed] [Google Scholar]
- 50.Jaramillo-Colorado B., Olivero-Verbej J., Stashenko E.E. Anti-quorum sensing activity of essential oils from Colombian plants. Nat. Prod. Res. 2012;26:1075–1086. doi: 10.1080/14786419.2011.557376. [DOI] [PubMed] [Google Scholar]