Figure 3.
MyoD Upregulation by Hypoxia Occurs via Sp1 Stimulation in Hypoxic EBs
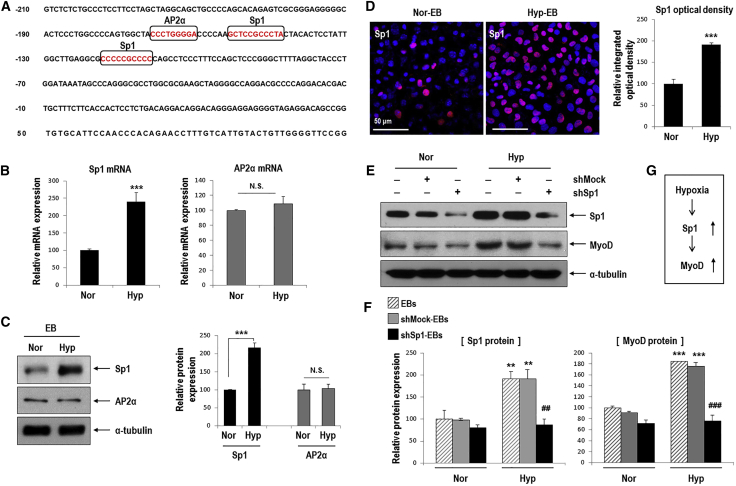
(A) The mouse MyoD putative promoter region was found to contain candidate Sp1- and AP2α-binding sites (marked by squares). Nucleotides are numbered relative to the translation start site of MyoD. (B and C) Sp1, but not AP2α, was remarkably increased in hypoxic EBs compared to normoxic EBs (B) Real-time PCR for Sp1 and AP2α mRNA (n = 5, ***p < 0.001). (C) Western blot for Sp1 and AP2α (left). Quantification of western blotting results (right; n = 3, ***p < 0.001). (D) Immunofluorescence staining of Hyp-EBs and Nor-EBs for Sp1 (red) and nuclei (blue) on day 3 after attachment (left). Magnification, 400×; scale bars, 50 μm. Quantitative data for the intensity of Sp1 fluorescence. At least three randomly selected fields were analyzed from three independent experiments (***p < 0.001). (E) Transient transfection of shSp1. ESCs were transfected with 1 μg shMock or shSp1 for 24 h, allowed to form EBs for 3 days under normoxia, and further incubated for 16 h under normoxic or hypoxic conditions. Sp1 protein was markedly reduced after transient transfection of shSp1 compared to that in the non-target shRNA control (shMock). Sp1 knockdown suppressed the increased expression of MyoD protein even under conditions of hypoxia. (F) Quantification of western blotting results for Sp1 and MyoD proteins (n = 3); **p < 0.01, ***p < 0.001 versus Nor-EBs. ##p < 0.01, ###p < 0.001 versus Hyp-shMock-EBs. (G) Sp1 was stimulated by hypoxia and then increased MyoD expression.