Figure 6.
Knockdown of Sp1 in Hypoxia-Primed EBs Diminishes Muscle Differentiation in an In Vivo Mouse Muscle Injury Model
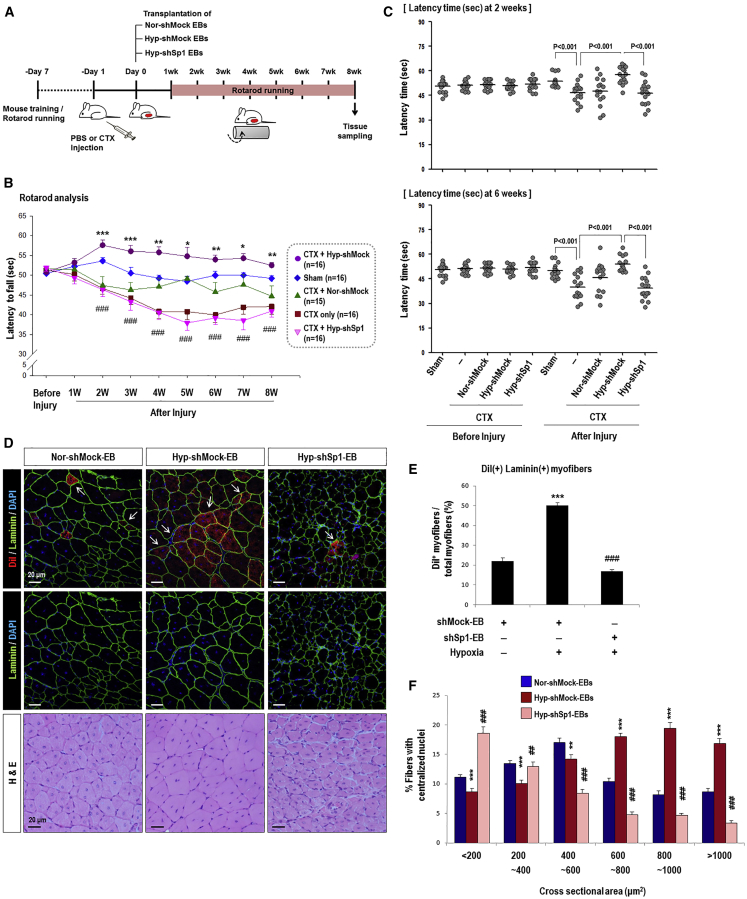
(A) Timetable of rota-rod analysis of mice with skeletal muscle injury. One day before cell transplantation, CTX was injected into the TA muscle (both legs) to induce muscle injury. We then monitored muscle power every week for 8 weeks in five groups: sham group with PBS injection (sham, n = 16), injury only group with CTX injection (CTX only, n = 16), injury and transplantation of normoxic shMock-EBs (CTX+Nor-shMock, n = 15), injury and transplantation of hypoxic shMock-EBs (CTX+Hyp-shMock, n = 16), and injury and transplantation of hypoxic shSp1-EBs (CTX+Hyp-shSp1, n = 16). (B and C) The time required for mice to fall off the rotating rod was measured every week for 8 weeks (B) and at 2 weeks and 6 weeks after cell transplantation (C) as shown; *p < 0.05, **p < 0.01, ***p < 0.001 versus Nor-shMock-EBs and ###p < 0.001 versus Hyp-shMock-EBs. (D) Cross-section of TA muscle was stained with H&E or immunostained for laminin 2α (green)/nuclei (blue) at 8 weeks after transplantation. Transplanted cells were pre-labeled with DiI (red). Most DiI fluorescence-positive red cells were regenerating myofibers with a central nucleus. Magnification, 200×; scale bars, 20 μm. Myofibers positive for DiI in the Hyp-shSp1 group were smaller than those in the Hyp-shMock group. (E) Quantification of DiI(+)/Laminin(+) myofibers, which was high in the Hyp-shMock-EB group but decreased in the Hy-shSp1-EB-transplanted TA muscle (n = 30; ***p < 0.001 versus Nor-shMock-EBs and ###p < 0.001 versus Hyp-shMock-EBs). (F) CSA of regenerating myofibers. TA muscle-transplanted cells were collected at 8 weeks after surgery and H&E staining and CSA (μm2) were measured. CSA of the regenerating myofibers in the Hyp-shSp1 group were significantly smaller than those in the Hyp-shMock group (n = 39; **p < 0.01, ***p < 0.001 versus Nor-shMock-EBs and ##p < 0.01, ###p < 0.001 versus Hyp-shMock-EBs).