Implications.
Magnetic resonance imaging is a very powerful approach to perform anatomical and functional imaging explorations.
Large animal models are perfectly suited for MRI studies using clinical devices and these tools are currently being developed and improved.
Here we highlight an example of MRI to demonstrate the efficacy of gene therapy in sheep models of Batten disease, a pediatric neurodegenerative disease.
Introduction
Imaging techniques used in experimental or medical fields are currently undergoing spectacular development. Whether based on the use of ultrasound (Doppler imaging), x-rays (computerized tomography, CT scan), or magnetic resonance imaging (MRI), they offer an unprecedented tool for anatomical or physiological explorations. In this review, we highlight the possibilities offered by MRI and its applications to large animal models in the context of preclinical research. MRI is a nonionizing imaging method that combines both radio-frequency electromagnetic pulses and a strong magnetic field. First, we describe the principles and potency of magnetic brain imaging to provide a baseline understanding of the principles underlying this technology as well as an appreciation of the benefits and limitations of its use. We also describe how these techniques are currently being applied to large animal models, especially for neurobiological research in sheep. Given that MRI approaches are still in their infancy with respect to animal models, the development of associated tools will certainly boost the application of MRI to large animal models. Finally, we illustrate the possibilities of the use of MRI in the case of a pediatric neurodegenerative disease (Batten disease) on sheep genetic models of proven validity, to explore the pathophysiological mechanisms involved, and the development of therapeutic strategies.
Magnetic Resonance Imaging Principles
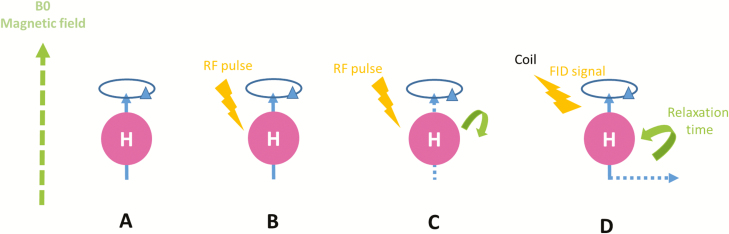
MRI is based on the absorption and emission of radio-frequency energy from specific atomic nuclei placed in a strong magnetic field. Hydrogens atoms (protons) are the most frequently used because they are highly abundant in water, fat, proteins, carbohydrates, and nucleic acids in tissues of living organisms. Protons (hydrogen nuclei) exhibit an intrinsic property of “spin” in which they rotate around their axes and being charged particles, this spin produces a small magnetic field. When outside of an external magnetic field, spins (protons) will point randomly in any direction, thus yielding a null magnetization moment. Placed in a strong magnetic field, B0, protons exhibit a weak tendency to align with the direction of B0 (Figure 1A). In addition, protons turn around B0 like gyroscopes (precession); thus, the axis of rotation traces out a circular path around the direction of the magnetic field at the Larmor frequency (ω0 = γB0). Together, the many spinning protons form a net magnetization, M, parallel to B0. An application of a radio-frequency (RF) pulse (Figure 1B), B1, perpendicular to B0, with a frequency equal to ω0, pushes M away from B0 (resonance, Figure 1C). Once the RF signal stops, M returns to equilibrium (relaxation) such that it is parallel again to B0. The net magnetization also processes around B0, and as it relaxes to equilibrium, it induces a signal (free-induction decay [FID]) which can be measured by a coil placed around the object being imaged and reconstructed to obtain three-dimensional (3D) gray-scale MR images (Figure 1D). Localization of the signal in the 3D space is ensured by magnetic field gradients. Finally, the acquisition parameters have to be chosen to correspond to the constraints of the spatial resolution (voxel size) and the desired signal quality (signal-to-noise ratio).
Figure 1.
Schematic representation of magnetic resonance imaging principles. (A) When placed in a strong magnetic field B0, protons align themselves parallel or antiparallel with the direction of B0. (B) The application of a RF pulse B1 perpendicular to B0 and at the same frequency of B0 allows the longitudinal magnetization to tilt away from B0 (resonance). (C) Once the RF signal stops, M returns to equilibrium (relaxation) such that it aligns again with B0. (D) During relaxation, protons lose energy by emitting a RF signal Free Induction Decay (FID) which can be measured by a coil placed around the object being imaged and subsequently reconstructed to obtain 3D gray-scale MR images.
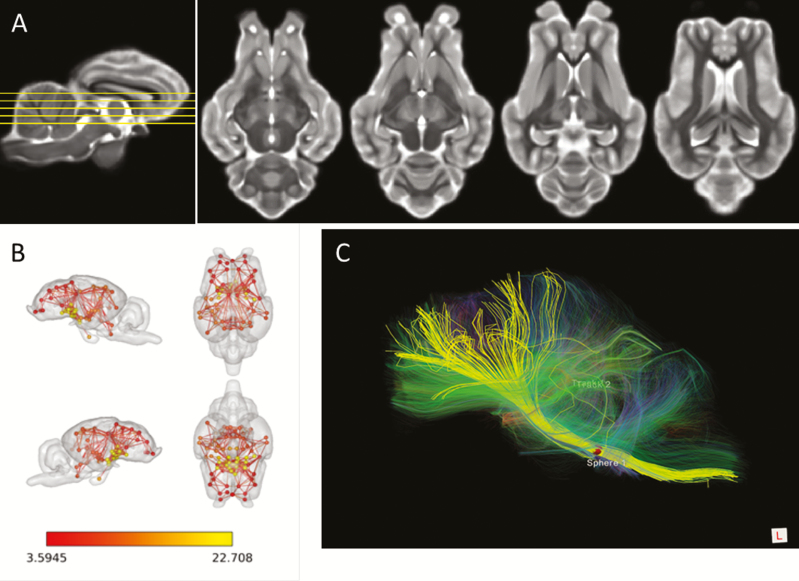
By nature, MRI is a highly multimodal imaging approach. First, MRI can be used to provide anatomical images (Figure 2A), which can express various contrasts depending on the parameters of the excitation used. This contrast is due to the various relaxation properties of the protons in the tissues. Among the major image contrasts are T1- and T2-weighted images, which reflect two different relaxation times (T1 longitudinal relaxation time vs. T2 transverse relaxation time). T1- and T2-weighted images can be easily differentiated by the appearance of fluids, which appear black on T1 in contrast to white on T2. Many other sequences are available to create various contrasts that can bring complimentary information to the anatomical question being explored. Furthermore, many contrast agents can be used to enhance the visualization of specific structures. For example, gadolinium is used routinely in tumor imaging to shorten T1 relaxation times, thereby enhancing image contrast.
Figure 2.
The multimodal potency of MRI. (A) T2 weighed MRI images of the sheep brain at four different dorso–ventral levels. (B) Resting-state networks defined using resting state fMRI and associated analysis in anesthetized sheep. Three internal subnetworks involving the frontal region (red nodes), the occipito–parietal region (orange nodes), and the diencephalic region (yellow nodes) can be defined. (C) Identification of some main fiber tracts (yellow) connecting the brainstem (right) to the frontal regions (left) and visualized by diffusion tensor imaging within the sheep brain.
Another important imaging technique relies on functional MRI approaches. Functional MRI (fMRI) is an indirect measure of brain activity which detects functional change associated with blood flow oxygenation. Indeed, the deoxyhemoglobin acts as an endogenous contrast agent which reduces the signal in T2-weighted images when its concentration increases. This modality relies on the fact that in the brain, neuronal activations are tightly coupled with cerebral hemodynamic responses and permit functional exploration of the brain during a task or at rest. In humans or in rodents, the main form of fMRI uses the blood-oxygen-level dependent (BOLD) contrast expressed in response to various stimulations such as a sensory stimulation or performance of a motor/cognitive task. In addition, “resting state” fMRI has become ubiquitous in recent years. This measures BOLD fluctuations in the absence of an overt task and investigates how spatially distinct regions of the brain are functionally connected. Application of this technique has identified many functional brain networks; the most well-described is the default mode network (DMN), a large-scale brain network of interacting structures which express a highly correlated neuronal activity (Figure 2B).
Diffusion-weighted MRI is another important type of MRI that allows the mapping of in vivo diffusion of water molecules in biological tissues, which reflects the interactions of water molecules with tissue components, such as macromolecules, fibers, and cell membranes. This technique can therefore reveal anatomical details about tissue architecture. In the field of neuroimaging, a special type of diffusion-weighted MRI called diffusion tensor imaging (DTI) is widely used to map the main white matter tracts of the brain (Figure 2C).
Finally, there is nuclear magnetic resonance (NMR) spectroscopy, sometimes shortened to MR spectroscopy, which is based on small changes in the intramolecular magnetic field around an atom because of the different influences of different adjacent atoms and thus small shifts in the resonance frequency, giving access to details of the bonding structure of specific molecules. This is usually used for the analysis and identification of monomolecular organic compounds, such as for the analysis of metabolic changes in a tissue. For example, MR spectroscopy is used clinically to grade tumor malignancy on the basis of their metabolic activity.
Use of MRI on Large Animal Models for Preclinical Research
Large “domestic” animal models (sheep, goats, pigs, etc.) are of interest for a range of fundamental, agricultural, and preclinical research purposes. Often they exhibit interesting features for preclinical research because their anatomy and physiology may be closer to humans than rodents. For example, the gross anatomy of pig or sheep brains is quite similar to that of primates, including humans. Brains from domestic animals show a high degree of cortical gyrification, in contrast to rodents which have a lissencephalic cortex. Pigs are monogastric and have a digestive system that is very close to humans both anatomically and physiologically. In this regard, such models could become an alternative to primates for specific fields, especially given the financial difficulties and ethical considerations associated with primate models.
Due to their evolutionary history, domestic animals also exhibit specific physiological regulations that can be complimentary to those observed in rodents. For example, small ruminants are seasonal breeders, a characteristic not found in many laboratory animals, which has important consequences for animal production (Malpaux et al., 2001). Sheep are also a valuable model to study the effects of early brain development and sexual differentiation on later adult life (Roselli and Stormshak, 2010). Finally, sheep give birth to precocial young which are fully developed at birth in contrast to rodents where young are highly immature. Such features allow the study of mother-young attachment processes, which are based on individual recognition processes (Nowak et al., 2011).
A relatively long lifespan is an additional characteristic of interest for experimental research on large animal models. Although this could be a drawback when considering the costs associated with their breeding and maintenance, it is clearly an advantage when one wants to explore fine developmental processes or the long-term development of pathological events and the effects of potential experimental/curative treatments. In addition, the fact that large animals are domesticated also implies that they have been selected for their social abilities and are accustomed to the presence of humans. Therefore, manipulation of domestic animals is relatively easy with limited stressful consequences. If necessary, habituation procedures can be performed routinely. Finally, due to their size, it is clear that specific sampling which necessitates a minimal amount of material can be performed much more easily on large animals. Sheep are one of the gold standard models used in neuroendocrinology due to the opportunity to perform serial blood (or cerebrospinal fluid) sampling at short intervals (each 10 to 20 min) and over very long durations (more than 24 h) without affecting the basal physiology of the animal. Such advantages allow exploration of the intricacies of endocrine dynamics. In addition, very fine surgical approaches cannot be performed easily in rodents. For example, cannulation of the portal blood system in the pituitary, which allows sampling of gonadotropin-releasing hormone directly at the site of its release (Clarke and Cummins, 1982), is not possible in rodents.
Without being exhaustive, the previous section shows quite clearly that large animal models have specific advantages when considering their use as experimental models. These assets provide an exciting potential for their use in preclinical or translational research and explains why their use is currently gaining popularity. Sheep are now used to study various pathophysiological conditions including (but not limited to) fetal growth during gestation (Harding and Bloomfield, 2004), epilepsy (Opdam et al., 2002), or stroke (Boltze et al., 2018).
MRI in large animal models presents clear advantages: 1) the methodology is not invasive; 2) MRI can be used for longitudinal studies, enabling the long-term follow-up on the effect of experimental manipulations; 3) MRI is highly multimodal, allowing acquisition of complimentary information (anatomical, functional, etc.) on the same animal within the same imaging session; 4) being noninvasive, MRI offers the possibility to use animals as their own controls, therefore limiting variability and increasing statistical power; and 5) it is possible to scan large animals (sheep, pigs, and goats) on current scanners, which are becoming more common in clinical and research environments.
For these reasons, the use of MRI in large animal models has been progressively increasing for fundamental or translational neuroscientific research (for example, Nuruddin et al., 2013). The first reported preclinical study used fMRI, combined with electroencephalography, for the localization of epileptic foci in the sheep brain (Opdam et al., 2002). Technical approaches combining anatomical MR images and surgical stereotactic access to the brainstem (Staudacher et al., 2014) or microelectrocorticography grid implantation in the mapping of sensory cortex were then developed in the sheep brain (Gierthmuehlen et al., 2014). More recently, Lee et al. (2015) imaged sheep brains using both fMRI and DTI protocols. Imaging studies have also been conducted in pigs. For example, fMRI has been used to evaluate maturation of the brain in minipigs from the early postnatal period to 6 mo of age to explore whether the sequence of development of the visual cortex and its spatial localization is similar to that in primates (Fang et al., 2006). MRI has been also used to track the recovery of ischemia following transplantation of induced pluripotent stem cell-derived neural stem cells in a pig model (Baker et al., 2017).
Although these studies have shown that large animal models may be appropriate for brain in vivo structural and functional MR assessments, they are usually illustrative/qualitative, rely on limited numbers of subjects, and are without any systematic and quantitative group analyses—because the necessary tools to process and analyze the images are lacking. In the field of neuroscience, brain analysis requires the registration of each single subject image into a common reference space, also known as a template image, which is generally associated with an atlas of brain structures. These tools allow valid analysis of images and facilitate: 1) statistical inferences over voxel intensities or shape variation of brain structures (e.g., voxel-based morphometry), 2) identification of brain structures activated during fMRI or positron emission tomography (PET), 3) functional connectivity analysis where brain structures can be used as regions of interest (ROI).
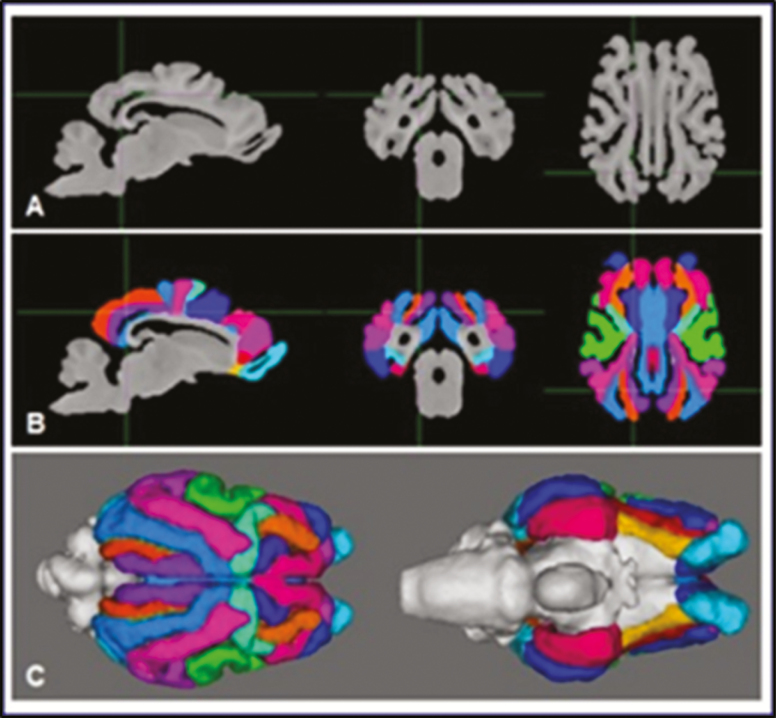
Such MRI tools were lacking but have become available recently. An MRI-based brain template for sheep was published in 2015 (Nitzsche et al., 2015), but it was incomplete, lacking, for example, the cerebellum or the olfactory bulbs. At the same time, we published another 3D MRI brain template with associated gray matter, white matter, and cerebrospinal fluid tissue probability maps (Ella and Keller, 2015; Ella et al., 2017). T1- and T2-weighted templates, as well as linear and nonlinear tissues probability maps, were computed from complete adult sheep brains acquired with native isotropic voxel sizes and at a higher resolution (Figure 3A). We also defined a stereotaxic atlas, where each brain structure is registered with a coordinate system common to the derived templates and tissue probability maps (Figure 3B and C). Finally, both cortical and subcortical structures were annotated and labeled using an existing histological atlas. Importantly, these tools (brain atlas, template, and probability maps) have also been developed in domestic pigs (Sauleau et al., 2009; Saikali et al., 2010) and Göttingen minipigs (Watanabe et al., 2001).
Figure 3.
Steps for the realization of a sheep brain template and atlas. (A) Acquisition of brain MR images and computation of a MR template of the sheep brain in T1-weighted contrast. (B) Segmentation of cortical areas overlaid on the template images. (C) Three-dimensional view of the segmented telencephalon.
Finally, it is worth mentioning that although most of the studies are still performed in a clinical environment, with strong constraints including the availability of equipment and sanitary precautions, dedicated MRI platforms are starting to emerge with high-quality holding facilities for small ruminants (sheep and goats), pigs, and even cattle. Two examples are the platform of the French National Agricultural Institute (INRA) in Nouzilly, France, which has been functioning since 2013, or the MRI platform which will open at the Roslin Institute/University of Edinburgh. These platforms provide access to other imaging techniques (e.g., CT scan), as well as other experimental approaches, including full scanning facilities and the possibility to perform studies in biological containment level 2. Such facilities are likely to increase within the main agricultural and veterinary academic institutions in the future, thereby facilitating the ability to perform fundamental or preclinical studies on large animal models. They also allow for optimized care of animals, in regard to ethical considerations, especially because the general public usually considers these domestic species as more sensitive than rodents. Indeed, the fact that all facilities (breeding and housing of animals, imaging, and surgical facilities) are physically colocated helps us to reduce animal stress associated with transport and additional manipulations.
An Example of Preclinical Research Using Sheep as a Model of a Pediatric Disease, Batten Disease
Batten disease is a group of rare genetic lysosomal diseases also known as the neuronal ceroid lipofuscinoses (NCL; Mole et al., 2011). These neurodegenerative lysosomal storage diseases predominantly affect children and are to date the most prevalent group of progressive childhood encephalopathies. Different forms of the disease are caused by mutations in various genes, with autosomal recessive mutations in CLN5 and CLN6 genes causing late-infantile variants (Kousi et al., 2012). The onset of late-infantile Batten disease usually occurs between 18 mo and 8 yr of age. Symptoms, which increase with age, include regression of motor and sensory functions, blindness, ataxia, and dementia, leading to premature death around 15 to 20 yr of age (Mole et al., 2011). Once diagnosed, the life expectancy of children is usually limited to a further 3 to 5 yr. To date, there is no curative treatment.
Understanding the pathophysiological mechanisms of Batten disease is still limited. The pathology is characterized by extensive brain atrophy, especially at the cortical level. In most forms, a clear accumulation of storage bodies, predominantly composed of subunit c of the mitochondrial ATP synthase, can be observed in most cells but this accumulation is unlikely to be responsible for the pathological mechanisms (Palmer et al., 2013).
Various naturally occurring animal models of Batten disease exist, including sheep models (Palmer et al., 2011, 2013; Bond, et al., 2013). Two main forms have been identified: a CLN5 form observed in Borderdale sheep (Frugier et al., 2008) and a CLN6 form in Merino and South Hampshire sheep (Tammen et al., 2006). Both CLN5 (CLN5−/−) and CLN6 affected (CLN6−/−) sheep share the main neuropathological features of the human disease, with progressive brain degeneration and a decline in motor and sensory function (blindness). Affected sheep decline to a humane endpoint before 20 to 24 mo of age. Both forms of NCL in sheep therefore represent excellent models to explore key questions such as: 1) when the disease starts and the timeline for progression of the disease; 2) why some brain regions are more affected than others; and most importantly 3) if therapeutic strategies can be established to stop or at least slow down disease progression.
In this context, MRI offers advantages to understand the relationship between the symptoms and the pathological mechanisms. Indeed, MRI is noninvasive by nature and is a perfect approach to perform longitudinal studies to track disease progression as well as the continuing effectiveness of therapeutic approaches such as viral-mediated gene therapy. Such trials extend over several years and require long-term monitoring of animals without prematurely sacrificing them for postmortem neuropathological evaluation.
A first approach in affected sheep used CT scanning with associated 3D reconstruction of the intracranial volume to track the brain neurodegeneration in CLN5−/− and CLN6−/− animals (Russell et al., 2018). Intracranial volume loss was minimal until 10 mo of age. After this age, the intracranial volume of both CLN5−/− and CLN6−/− animals reduced rapidly while unaffected animals maintained a stable intracranial volume. In addition, the intracranial volume changes reflected the previously established regionality of the pathology (Oswald et al., 2008).
Although the CT scan is adapted to image “hard” tissues (skull bone), it provides limited information on gray matter–white matter and other soft tissue delineation in the brain. In contrast, MRI allows exploration of soft tissues and can therefore be used to track the neurodegenerative process in more detail. In a preliminary study, Sawiak et al. (2015) followed the degenerative process in CLN6−/− sheep during the terminal stages of disease (17–22 mo). They validated the use of longitudinal MRI to study the dynamics of progressive neurodegeneration and confirmed the pronounced cortical atrophy in affected sheep. Significant atrophy was also observable in other brain regions such as the amygdala and the basal ganglia at the terminal stage of the disease. Importantly, the approach was sensitive enough to measure significant changes in the cortex over a 5-mo period, even after around 40% of the cortical volume was already lost at the beginning of the study.
Recently, Mitchell et al. (2018, Figure 4) aimed to characterize disease progression in CLN5−/− Borderdale sheep, and most importantly, to track and monitor the long-term efficacy of CLN5 gene therapy. Two viral vector platforms were used: single-stranded adeno-associated viral serotype 9 (ssAAV9) or lentivirus. Both vectors prevented the stereotypical disease development when administered by intracerebroventricular injection to presymptomatic lambs at 2 to 4 mo of age. The only clinical sign observed was delayed-onset visual loss, occurring between 20 and 24 mo. Despite this visual loss, the treated sheep were phenotypically normal at 27 mo of age, having normal social relationships. When tested in an open field, they showed no sign at all of the behavioral and neurological signs usually observed in the advanced stages of disease. One ssAAV9-treated animal has been retained until 62 mo old, which is 3 times older than the normal lifespan of untreated CLN5−/− sheep. Because gene therapy is more likely to begin after clinical diagnosis is established, a self-complementary AAV9-CLN5 was injected into 7-mo-old CLN5−/− sheep in a second trial (Mitchell et al., 2018). Consistent with the first trial, this treatment stopped progression of the disease, allowed maintenance of cognitive abilities, and the survival of these animals much exceeded their natural lifespan.
Figure 4.
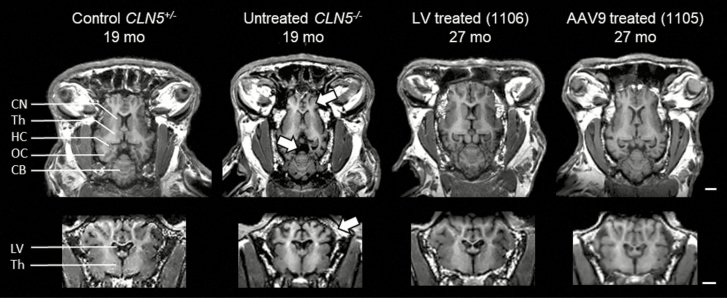
T1-weighted MRI images showing the preservation of neuroanatomical structures after gene therapy in CLN5−/− sheep. T1-weighted MRI images in the horizontal (top) or coronal (bottom) views from 19-mo old control or affected sheep compared with much older (27 mo old) sheep treated by gene therapy with lentivirus (LV) or single-stranded adeno-associated viral serotype 9 (AAV9) before the development of clinical signs. The treatments protected from the high neuronal atrophy observed in affected animals because either vector preserved neuroanatomical structures, protecting against the profound cortical atrophy (top arrow), prominent ventricular enlargement (center arrow), and cranial thickening (bottom arrow) seen in the untreated CLN5−/− animals. CB = cerebellum; CN = caudate nucleus; HC = hippocampus; LV = lateral ventricles; OC = occipital cortex; Th = thalamus. By permission from Elsevier (original publication: Mitchell et al. (2018), Molecular Therapy, 26(10): 2366–2378).
Longitudinal CT and MRI were used to track and monitor the brain volume and architecture. Although untreated CLN5−/− sheep showed a massive shrinkage of the brain (Mitchell et al., 2018), treated CLN5−/− sheep showed no sign of cerebral atrophy, with preservation of brain structures and volumes. MRI of sheep treated before development of clinical signs confirmed that CLN5 gene therapy afforded complete protection against the profound cortical atrophy, stereotypical ventricular enlargement, and cranial thickening.
Postmortem histological analysis demonstrated widespread expression of the CLN5 protein in the brain and spinal cord in all treated sheep, regardless of the vector used. CLN5 immunoreactivity was especially evident in the cerebral cortex and hippocampus of treated sheep, and transduced cells were morphologically neuronal-like. In contrast, there was no CLN5 protein expression in the untreated CLN5−/− sheep brain. As a whole, these data demonstrate the efficacy of CLN5 gene therapy, using three different viral vectors, and provide a strong rationale for clinical translation to CLN5-affected human patients. They also highlight the validity of MRI approaches and the use of the sheep models of Batten disease for preclinical studies.
Conclusion
The principles and advantages of the use of MRI in large animal models for preclinical purposes are highlighted in this review. Large animals present characteristics that make them more relevant than many other smaller animals for a range of applications, thus increasing the predictive values of the results obtained. Development of useful large animal models, advanced MRI to assess the brain in vivo, and newly developed analytical tools provide an incredibly powerful multimodal, noninvasive, and ethical framework with which to investigate a range of questions of importance to fundamental, agricultural, and preclinical research.
Interestingly, the increased access and use of MRI in large animal models highlight paradoxically our lack of fundamental physiological knowledge of these species. If we take the brain as an example, it is clear that deep brain structures have been relatively well described. However, many other regions (cortical regions) remained poorly explored at both the functional and anatomical levels. For example, there is still no functional map for domestic animals that is equivalent to the Brodmann’s area descriptions in human and nonhuman primates. As a result, localization and interpretation of activations identified by functional imaging remain somewhat imprecise. This limitation is also an opportunity: the development of imaging studies on large animal models represents a unique chance to provide new and original knowledge regarding the physiology of these animals, which can be used for the development of new applications in the field of animal science.
About the Authors

Arsene Ella has a PhD in biomedical engineering with expertise in medical image processing. He started his career by characterizing the human myocardium in SPECT and positron emission tomography (PET) imaging using the thin-plate spline warping. He moved to analysis of the human brain by investigating fDOPA kinetics and brain compartments in PET. Then, he implemented a new technique of correction of physiological noises in functional magnetic resonance imaging (fMRI). In collaboration with Dr Matthieu Keller, they developed the first complete 3D high-resolution probabilistic template and atlas of the sheep brain from MR images.

David Barrière’s scientific interests are related to the understanding of brain functions in health and disease through magnetic resonance imaging (MRI). During his PhD and post-doctoral experiences, he studied the consequences of chronic pain and chronic stress on brain function in rodents using both functional and structural MRI. He also developed methodologies, templates, and reference atlases for MRI analysis of the rodent brain. He conducted collaborative research with Dr Matthieu Keller on large animal models to characterize the neural networks responsible for sexual behavior in mammals.

Hans Adriaensen is a Research Engineer and is in charge of the 3T MRI scanner and the CT scanner on the CIRE Platform at INRA, Centre Val de Loire. He has been scanning large animals (sheep, calves, horses, and chickens) in vivo, since 2012 by adapting the machines to those animal models. His goal is to implement new imaging methods on the MRI and CT for researchers to address important biological questions.

Professor David N. Palmer, Lincoln University, studies sheep models of Batten disease (ceroid lipofuscinosis, NCLs) and inherited childhood neurodegenerative diseases. Sheep are an ideal model to study human disease and treatment responses. Having determined the pathogenic cascade, we are now trialing intraventricular viral-mediated gene therapy which has increased the lifetime of affected sheep over three-fold. He is also developing a suite of in vivo tests to determine therapy efficacy, including fine detail MRI monitoring.
Tracy Melzer is a medical physicist with subspecialty expertise in neuroimaging. He is interested in the application of multimodal imaging (including MRI and PET) to study a number of different diseases and conditions, including cognitive impairment in Parkinson’s disease, Alzheimer’s disease, child development, multiple sclerosis, brain injury, and more recently, large animal models of disease.

Nadia Mitchell is a New Zealand neuroscientist, working as a Postdoctoral Fellow at the University of Otago (Christchurch) and Adjunct Lecturer at Lincoln University. Her research has centered on two naturally occurring ovine models of Batten disease. She earned a PhD in 2016 by developing translational viral-mediated gene therapies in sheep. She combines longitudinal in vivo behavioral and neurological tests and quantifiable neuroimaging techniques in the sheep with postmortem neuropathological studies to monitor therapeutic efficacy and disease amelioration.

Matthieu Keller is a CNRS research director working in the fields of behavioral neuroendocrinology and reproductive physiology in rodents, but also a variety of large animal models, including sheep, goats, and horses. He has recently developed the use of MRI in such animal models, and associated tools including a brain template, atlas, and associated tissue probability maps in sheep.
Literature Cited
- Baker E.W., Platt S.R., Lau V.W., Grace H.E., Holmes S.P., Wang L., Duberstein K.J., Howerth E.W., Kinder H.A., Stice S.L., . et al. 2017. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci. Rep. 7:10075. doi: 10.1038/s41598-017-10406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltze J., Ferrara F., Hainsworth A.H., Bridges L.R., Zille M., Lobsien D., Barthel H., Mc Leod D.D., Grässer F., Pietsch S., . et al. 2018. Lesional and perilesional tissue characterization by automated image processing in a novel gyrencephalic animal model of peracute intracerebral hemorrhage. J. Cereb. Blood Flow Metab., in press. doi: 10.1177/027/1678X18802119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Holthaus S.M., Tammen I., Tear G., and Russell C.. . 2013. Use of model organisms for the study of neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta 1832:1842–1865. doi: 10.1016/j.bbadis.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Clarke I.J., and Cummins J.T.. . 1982. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111:1737–1739. doi: 10.1210/endo-111-5-1737 [DOI] [PubMed] [Google Scholar]
- Ella A., Delgadillo J.A., Chemineau P., and Keller M.. . 2017. Computation of a high-resolution MRI 3D stereotaxic atlas of the sheep brain. J. Comp. Neurol. 525:676–692. doi: 10.1002/cne.24079 [DOI] [PubMed] [Google Scholar]
- Ella A., and Keller M.. . 2015. Construction of an MRI 3D high resolution sheep brain template. Magn. Reson. Imaging 33:1329–1337. doi: 10.1016/j.mri.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Fang M., Li J., Rudd J.A., Wai S.M., Yew J.C., and Yew D.T.. . 2006. fMRI mapping of cortical centers following visual stimulation in postnatal pigs of different ages. Life Sci. 78:1197–1201. doi: 10.1016/j.lfs.2005.06.030 [DOI] [PubMed] [Google Scholar]
- Frugier T., Mitchell N.L., Tammen I., Houweling P.J., Arthur D.G., Kay G.W., van Diggelen O.P., Jolly R.D., and Palmer D.N.. . 2008. A new large animal model of CLN5 neuronal ceroid lipofuscinosis in borderdale sheep is caused by a nucleotide substitution at a consensus splice site (c.571 +1G>A) leading to excision of exon 3. Neurobiol. Dis. 29:306–315. doi: 10.1016/j.nbd.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierthmuehlen M., Wang X., Gkogkidis A., Henle C., Fischer J., Fehrenbacher T., Kohler F., Raab M., Mader I., Kuehn C., . et al. 2014. Mapping of sheep sensory cortex with a novel microelectrocorticography grid. J. Comp. Neurol. 522:3590–3608. doi: 10.1002/cne.23631 [DOI] [PubMed] [Google Scholar]
- Harding J.E., and Bloomfield F.H.. . 2004. Prenatal treatment of intrauterine growth restriction: lessons from the sheep model. Pediatr. Endocrinol. Rev. 2:182–192. [PubMed] [Google Scholar]
- Kousi M., Lehesjoki A.E., and Mole S.E.. . 2012. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum. Mutat. 33:42–63. doi: 10.1002/humu.21624 [DOI] [PubMed] [Google Scholar]
- Lee W., Lee S.D., Park M.Y., Foley L., Purcell-Estabrook E., Kim H., and Yoo S.S.. . 2015. Functional and diffusion tensor magnetic resonance imaging of the sheep brain. BMC Vet. Res. 11:262. doi: 10.1186/s12917-015-0581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpaux B., Migaud M., Tricoire H., and Chemineau P.. . 2001. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythms 16:336–347. doi: 10.1177/074873001129002051 [DOI] [PubMed] [Google Scholar]
- Mitchell N.L., Russell K.N., Wellby M.P., Wicky H.E., Schoderboeck L., Barrell G.K., Melzer T.R., Gray S.J., Hughes S.M., and Palmer D.N.. . 2018. Longitudinal in vivo monitoring of the CNS demonstrates the efficacy of gene therapy in a sheep model of CLN5 batten disease. Mol. Ther. 26:2366–2378. doi: 10.1016/j.ymthe.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole S.E., Williams R.E., and Goebel H.H.. . 2011. The neuronal ceroid lipofuscinoses (Batten disease). 2nd ed. Oxford (UK): Oxford University Press. [Google Scholar]
- Nitzsche B., Frey S., Collins L.D., Seeger J., Lobsien D., Dreyer A., Kirsten H., Stoffel M.H., Fonov V.S., and Boltze J.. . 2015. A stereotaxic, population-averaged T1W ovine brain atlas including cerebral morphology and tissue volumes. Front. Neuroanat. 9:69. doi: 10.3389/fnana.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R., Keller M., and Lévy F.. . 2011. Mother-young relationships in sheep: a model for a multidisciplinary approach of the study of attachment in mammals. J. Neuroendocrinol. 23:1042–1053. doi: 10.1111/j.1365-2826.2011.02205.x [DOI] [PubMed] [Google Scholar]
- Nuruddin S., Bruchhage M., Ropstad E., Krogenæs A., Evans N.P., Robinson J.E., Endestad T., Westlye L.T., Madison C., and Haraldsen I.R.H.. . 2013. Effects of peripubertal gonadotropin-releasing hormone agonist on brain development in sheep--a magnetic resonance imaging study. Psychoneuroendocrinology 38:1994–2002. doi: 10.1016/j.psyneuen.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Opdam H.I., Federico P., Jackson G.D., Buchanan J., Abbott D.F., Fabinyi G.C., Syngeniotis A., Vosmansky M., Archer J.S., Wellard R.M., . et al. 2002. A sheep model for the study of focal epilepsy with concurrent intracranial EEG and functional MRI. Epilepsia 43:779–787. doi: 10.146/j.1528-1157.2002.04202.x [DOI] [PubMed] [Google Scholar]
- Oswald M.J., Palmer D.N., Kay G.W., Barwell K.J., and Cooper J.D.. . 2008. Location and connectivity determine gabaergic interneuron survival in the brains of South Hampshire sheep with CLN6 neuronal ceroid lipofuscinosis. Neurobiol. Dis. 32:50–65. doi: 10.1016/j.nbd.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D.N., Barry L.A., Tyynelä J., and Cooper J.D.. . 2013. NCL disease mechanisms. Biochim. Biophys. Acta 1832:1882–1893. doi: 10.1016/j.bbadis.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Palmer D.N., Tammen I., Drögemüller C., Johnson G.S., Katz M.L., and Lingass F.. . 2011. Large animal models. In: Mole S.E., Williams R.R., and Goebel H.H., editors. The neuronal ceroid Lipofuscinoses (Batten disease). 2nd ed. Oxford (UK): Oxford University Press; p. 284–320. [Google Scholar]
- Roselli C.E., and Stormshak F.. . 2010. The ovine sexually dimorphic nucleus, aromatase, and sexual partner preferences in sheep. J. Steroid Biochem. Mol. Biol. 118:252–256. doi:10.1016/j.jsbmb.2009.10.009. doi: 10.1093/med/9780199590018.001.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell K.N., Mitchell N.L., Anderson N.G., Bunt C.R., Wellby M.P., Melzer T.R., Barrell G.K., and Palmer D.N.. . 2018. Computed tomography provides enhanced techniques for longitudinal monitoring of progressive intracranial volume loss associated with regional neurodegeneration in ovine neuronal ceroid lipofuscinoses. Brain Behav. 8:e01096. doi: 10.1002/brb3.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikali S., Meurice P., Sauleau P., Eliat P.A., Bellaud P., Randuineau G., Vérin M., and Malbert C.H.. . 2010. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J. Neurosci. Methods 192:102–109. doi: 10.1016/j.jneumeth.2010.07.041 [DOI] [PubMed] [Google Scholar]
- Sauleau P., Lapouble E., Val-Laillet D., and Malbert C.H.. . 2009. The pig model in brain imaging and neurosurgery. Animal 3:1138–1151. doi: 10.1017/S1751731109004649 [DOI] [PubMed] [Google Scholar]
- Sawiak S.J., Perumal S.R., Rudiger S.R., Matthews L., Mitchell N.L., McLaughlan C.J., Bawden C.S., Palmer D.N., Kuchel T., and Morton A.J.. . 2015. Rapid and progressive regional brain atrophy in CLN6 batten disease affected sheep measured with longitudinal magnetic resonance imaging. PLoS ONE 10:e0132331. doi: 10.1371/journal.pone.0132331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudacher A., Oevermann A., Stoffel M.H., and Gorgas D.. . 2014. Validation of a magnetic resonance imaging guided stereotactic access to the ovine brainstem. BMC Vet. Res. 10:216. doi: 10.1186/s12917-014-0216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammen I., Houweling P.J., Frugier T., Mitchell N.L., Kay G.W., Cavanagh J.A., Cook R.W., Raadsma H.W., and Palmer D.N.. . 2006. A missense mutation (c.184C>T) in ovine CLN6 causes neuronal ceroid lipofuscinosis in merino sheep whereas affected South Hampshire sheep have reduced levels of CLN6 mRNA. Biochim. Biophys. Acta 1762:898–905. doi: 10.1016/j.bbadis.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Andersen F., Simonsen C.Z., Evans S.M., Gjedde A., and Cumming P.; DaNeX Study Group 2001. MR-based statistical atlas of the göttingen minipig brain. Neuroimage 14:1089–1096. doi: 10.1006/nimg.2001.0910 [DOI] [PubMed] [Google Scholar]