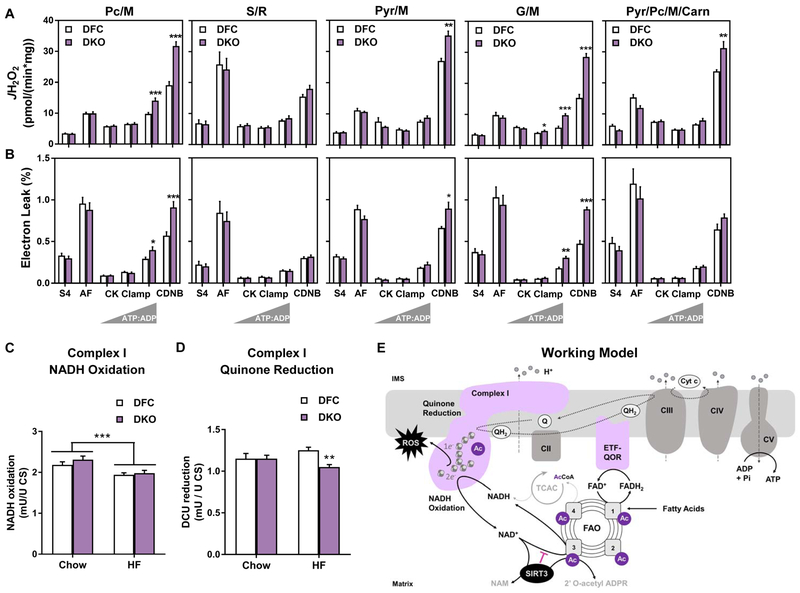
Figure 7. Disruption of acetyl-lysine turnover in DKO mitochondria exacerbates diet-induced mitochondrial H2O2 emissions and electron leak under physiologically relevant energetic conditions.
Mitochondria were isolated from skeletal muscles of DFC and DKO mice fed a high fat (HF) diet. (A) H2O2 emissions (JH2O2) and (B) electron leak, expressed as a percentage of oxygen flux (JH2O2/JO2 x 100 = % Electron Leak) were measured in mitochondria fueled by palmitoylcarnitine+malate (Pc/M), succinate/rotenone (S/R), pyruvate+malate (Pyr/M), glutamate/malate (G/M), or Pyr/M+Pc+free carnitine (Carn). Right triangles represent increasing concentrations of ATP relative to ADP (ATP:ADP) during the CK clamp, resulting in reciprocal changes in energy demand and thus JO2. Data represent mean ±SEM. Muscle lysates were used to assay (C) Complex I NADH oxidation and (D) Complex I quinone reduction. (E) Working model. Sirt3 consumes NAD regenerated by Complex I, which raises the local NADH/NAD+ redox charge and thereby inhibits the penultimate step in fatty acid oxidation (FAO). In DKO mice fed a high fat (HF) diet, Complex I is hyperacetylated at K42 of the NDUSF3 subunit, possibly perturbing electron transfer and/or Q reductase activity. The combination of disinhibited FAO and altered redox control promotes electron leak and production of reactive oxygen species (ROS). (A and B) N=5–10 per group, analyzed by Student’s t-test. *P≤0.05, **P≤0.01, ***P≤0.001. (C) N= 8 per group, (D) N=13 per group, data shown in (C and D) were analyzed by Two-way ANOVA with Tukey post-hoc testing, in (C) *** represents a main effect of diet. (D) ** represents a difference between DFC and DKO after Two-way ANOVA with Tukey post-hoc testing. N represents biological replicates.