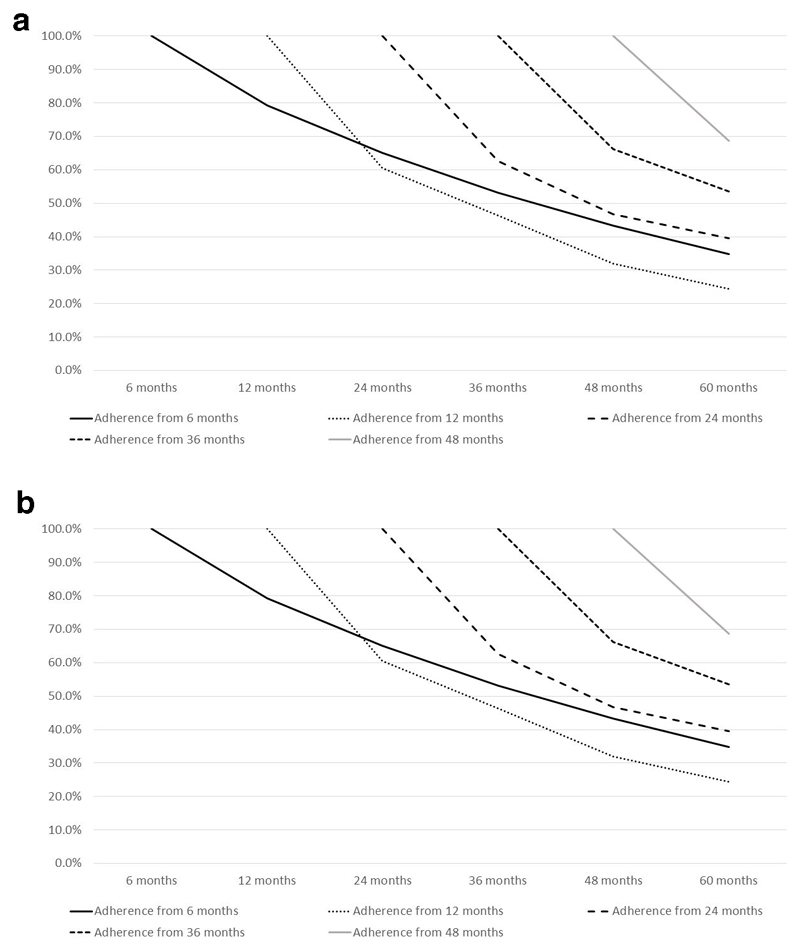
Figure 3.
a: Anti-osteoporosis medication (AOM) adherence over the 5 year duration of the SCOOP trial in study participants who initiated treatment, and who had not experienced a fracture between baseline and commencement of medication. (Calculated as the percent study participants who remained on AOM at each subsequent timepoint having initiated treatment at each index timepoint.)
b: Anti-osteoporosis medication (AOM) adherence over the 5 year duration of the SCOOP trial in study participants who initiated treatment after the occurrence of a fracture post-baseline. (Calculated as the percent study participants who remained on AOM at each subsequent timepoint having initiated treatment at each index timepoint.)