Abstract
Objective
To compare the efficacy of subthalamic nucleus (STN) and globus pallidus internus (GPi) deep brain stimulation (DBS) on reducing levodopa‐induced dyskinesia (LID) in Parkinson’s disease, and to explore the potential underlying mechanisms.
Methods
We retrospectively assessed clinical outcomes in 43 patients with preoperative LID who underwent DBS targeting the STN (20/43) or GPi (23/43). The primary clinical outcome was the change from baseline in the Unified Dyskinesia Rating Scale (UDysRS) and secondary outcomes included changes in the total daily levodopa equivalent dose, the drug‐off Unified Parkinson Disease Rating Scale Part Ⅲ at the last follow‐up (median, 18 months), adverse effects, and programming settings. Correlation analysis was used to find potential associated factors that could be used to predict the efficacy of DBS for dyskinesia management.
Results
Compared to baseline, both the STN group and the GPi group showed significant improvement in LID with 60.73 ± 40.29% (mean ± standard deviation) and 93.78 ± 14.15% improvement, respectively, according to the UDysRS score. Furthermore, GPi‐DBS provided greater clinical benefit in the improvement of dyskinesia (P < 0.05) compared to the STN. Compared to the GPi group, the levodopa equivalent dose reduction was greater in the STN group at the last follow‐up (43.81% vs. 13.29%, P < 0.05). For the correlation analysis, the improvement in the UDysRS outcomes were significantly associated with a reduction in levodopa equivalent dose in the STN group (r = 0.543, P = 0.013), but not in the GPi group (r = −0.056, P = 0.801).
Interpretation
Both STN and GPi‐DBS have a beneficial effect on LID but GPi‐DBS provided greater anti‐dyskinetic effects. Dyskinesia suppression for STN‐DBS may depend on the reduction of levodopa equivalent dose. Unlike the STN, GPi‐DBS might exert a direct and independent anti‐dyskinesia effect.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by progressive motor disability that includes bradykinesia, rigidity, resting tremor, and gait dysfunction along with a spectrum of non‐motor symptoms that include autonomic, cognitive, mood, sleep, and sensory symptoms.1 Despite advancements in medical treatment, after several years of levodopa use, some patients in the advanced stage of PD develop unexpected and serious disabling motor complications.2, 3 Levodopa‐induced dyskinesia (LID) is the most common complication for PD patients using long‐term drug therapy. LID encompasses a variety of different hyperkinetic phenomenologies including chorea, dystonia, stereotypies, and akathisia.3 Non‐physiological pulsatile dopamine release and synaptic pruning in the previously denervated striatum may both play critical roles in the development of LID.4 Previous studies have shown that the rate of dyskinesia in patients with over 5 years of drug therapy is approximately 30–50%,5, 6 while the incidence can reach 60–90% for patients with more than 10 years of drug therapy.7, 8 Dyskinesia can interfere with daily living activities, and impose a heavy burden on families.2, 9
Reducing the dosage or adjusting the regimen of medication might be effective in controlling dyskinesia,2, 3, 6, 10 but often leads to poor control of parkinsonism. Therefore, the “therapeutic window” is drastically narrowed, as such striking the balance in medication dose between dyskinesia and parkinsonian symptoms is difficult.11, 12, 13 Therefore, deep brain stimulation (DBS) has been established as an effective and safe alternative treatment. Both the subthalamic nucleus (STN) and the globus pallidus internus (GPi) are well‐established surgical targets for DBS treatment.8, 14 The STN and the GPi have been reported effective for the control of dyskinesia.14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Furthermore, while many studies have compared the GPi and STN‐DBS with respect to typical symptoms of PD, comparison for the control of dyskinesia has been scarcely reported and long‐term follow‐up focused on LID is even more rare.12, 22 No consensus has been reached about which stimulation target is optimal for the control of dyskinesia in PD patients. Up to now, the underlying mechanisms responsible for the reduction of dyskinesia after DBS are unknown. Generally, management of dyskinesia with STN‐DBS is mainly achieved by decreasing medication with a relatively greater reduction in levodopa equivalent daily dose (LED).14, 23, 24 Compared to STN‐DBS, GPi‐DBS provides a marginal reduction in dopaminergic dose, but it may have a direct effect on dyskinesia.25 However, the underlying mechanisms are still not completely understood.
Considering the importance of dyskinesia and the lack of literature surrounding it, we conducted a retrospective study in patients with pre‐operative dyskinesia in our center, and used the Unified Dyskinesia Rating Scale (UDysRS) to capture the presence and severity of dyskinesia after DBS treatment. We aimed to compare the efficacy of STN and GPi‐DBS in treating LID, as well as to explore possible mechanisms related to the relief of dyskinesia with DBS.
Methods
Subjects
We retrospectively assessed the clinical outcomes of patients with pre‐operative LID who underwent DBS targeting the GPi or the STN at the Beijing Tiantan Hospital from August 2015 to August 2018. Patients with advanced idiopathic PD diagnosed based on the UK bank criteria,26 who suffered from disabling LID before surgery were screened. A total of 61 patients were identified. Patients who previously underwent thalamotomy or pallidotomy, and those lost to follow‐up were excluded. Eventually, 43 patients were included in the study, with 20 patients treated with STN‐DBS and 23 patients with GPi‐DBS, respectively, as described in Table 1. Target selection was performed randomly as current literature supports similar motor benefit between two targets with minor differences. All patients received bilateral stimulation. All PD patients were informed and gave written consent, and the study was approved by the ethics committee at Beijing Tiantan Hospital.
Table 1.
Baseline demographic and clinical information of 43 patients.
| Variable | STN | GPi | P value |
|---|---|---|---|
| Number | 20 | 23 | – |
| Male sex – N (%) | 4 (20) | 11 (47.83) | 0.056 |
| Age of onset (year) | 48.70 ± 7.67 | 48.30 ± 9.48 | 0.874 |
| Course of disease before surgery (year) | 10.95 ± 4.79 | 12.13 ± 3.82 | 0.251 |
| Age at DBS (year) | 59.65 ± 9.11 | 60.43 ± 8.44 | 0.903 |
| Levodopa equivalent dose (mg/day) | 827.04 ± 376.39 | 981.76 ± 426.09 | 0.184 |
| Dyskinesia score (UDysRS) | 27.5 (14–76) | 34 (7–63) | 0.257 |
| Drug‐off UPDRS‐Ⅲ score | 47.85 ± 14.95 | 50.68 ± 15.36 | 0.415 |
| Hoehn‐Yahr stage, % | |||
| 2 | 1 | 2 | – |
| 2.5 | 7 | 6 | – |
| 3 | 10 | 12 | – |
| 4 | 2 | 3 | – |
| Follow‐up time (month) | 21.60 ± 8.79 | 18.26 ± 8.38 | 0.202 |
Values are presented as mean ± standard deviation (SD) or median (range). P values for comparisons between groups based on analysis of Wilcoxon rank‐sum tests and Chi‐Squared Test (sex). DBS, deep brain stimulation; GPi, globus pallidus interna; STN, subthalamic nucleus; UPDRS‐Ⅲ, Unified Parkinson’s Disease Rating Scale Part Ⅲ; UDysRS, the Unified Dyskinesia Rating Scale.
Surgical procedure
DBS electrode implantation was performed under local anesthesia, using a Leksell microstereotactic system (Elekta Instrument AB, Stockholm, Sweden). Intra‐operative single unit recordings and high frequency stimulation testing were used to assess the optimal location for permanent electrode implantation. Quadripolar electrodes (3387 Medtronic, Minneapolis, MN for GPi target; 3389 Medtronic for STN target) were implanted in all patients. The GPi target coordinates for the lower contact were 2 mm anterior to the mid commissural point (MCP), 18–22 mm lateral to the anterior commissure – posterior commissure (AC‐PC) and 6–9 mm below the inter‐commissural line. The STN target coordinates for the lower contact were 2–3 mm posterior to the MCP, 12–14 mm lateral to AC‐PC and 4–6 mm below the inter‐commissural line. The electrodes were then connected to an implantable pulse generator (IPG) implanted in the subclavicular area under general anesthesia. Next, post‐operative CT was performed to exclude intracranial hemorrhage and to verify the exact location of the electrodes by merging with the preoperative MR images. The IPG was turned on 1 month after the operation. Following the surgery, each patient underwent a regular adjustment of stimulation settings and medication until optimal control of symptoms was established. Typically, the patients’ improvement stabilized 6 months after surgery. Patients had visits at least every 6 months for clinical assessments and adjustment of stimulation settings and medication. All post‐operative adjustment of DBS parameter settings was performed while subjects were in an off‐medication state after at least 12 hours without taking any dopaminergic medications.
Outcome measures
The primary outcome measure was the change in the UDysRS scores, which can simultaneously be used to evaluate several subjective and objective symptoms of dyskinesia.27, 28 Items of the UDysRS scale were summarized into four subdomains: on‐dyskinesia, off‐dystonia, impairment and disability, in which the first two parts are historical and the latter two parts are objective. The secondary outcome measures included LED reduction, the drug‐off Unified Parkinson Disease Rating Scale Part Ⅲ (UPDRS‐Ⅲ) score changes, adverse effects, and programming settings. The dopaminergic treatment regimen was recorded as LED, according to the following convention: 100 mg of standard L‐dopa = 130 mg controlled‐release L‐dopa = 1 mg pergolide = 1 mg lisuride = 10 mg bromocriptine = 1 mg apomorphine = 60 mg piribedil = 6 mg ropinirole.19 All patients' scores of clinical scales were evaluated 1–2 weeks pre‐operatively and then every 6 months after DBS stimulation. The clinical improvement was computed as ([(Pre‐scores − Pos‐scores)/Pre‐scores] × 100%).
Statistical analyses
Demographic information was analyzed using descriptive analysis methods, and is described as mean ± standard deviation (SD) or median with range. Mann–Whitney U tests were used to compare groups for age, course of disease, etc., and Chi square test was used to compare the groups by sex. A paired‐sample nonparametric Wilcoxon signed‐rank test was used to determine whether there was a significant difference between the clinical scale scores at baseline and last follow‐up. Spearman correlation analysis was used to identify factors that associated with LID outcomes. These factors included age of onset, age at DBS, course of disease before surgery, pre‐operative drug‐off UPDRS‐Ⅲ, LID, LED, pre‐operative drug improvement rate, and drug‐off UPDRS‐Ⅲ and LED changes after surgery. The statistical significance threshold was fixed at P < 0.05. Statistical analysis was performed with IBM SPSS (version 20.0; SPSS Inc, Chicago, IL).
Results
Patient characteristics
Demographic characteristics of patients are summarized in Table 1. There were 20 STN‐DBS patients and 23 GPi‐DBS patients. The two groups of patients were similar with respect to sex (P = 0.056), age of onset (P = 0.874), course of disease before surgery, (P = 0.251), age at DBS (P = 0.903), drug‐off UPDRS‐Ⅲ score (P = 0.415), LED (P = 0.184), LID (P = 0.257) and follow‐up time (P = 0.202). The mean follow‐up in our cohort was 21.60 ± 8.79 months for STN‐DBS and 18.26 ± 8.38 months for GPi‐DBS.
As shown in Tables S1 and S2, the coordinates of left and right STN were (X, Y, Z, 112.04 ± 2.54, 96.20 ± 4.58, 104.33 ± 6.49) and (88.70 ± 2.25, 96.32 ± 4.80, 104.09 ± 6.42), while the coordinates of GPi were (119.39 ± 3.04, 100.83 ± 3.66, 106.84 ± 5.40) and (80.27 ± 2.92, 100.93 ± 4.21, 107.02 ± 5.27).
Primary outcome – UDysRS scale
Compared with baseline, there was a significant reduction in UDysRS scores after STN stimulation (from 27.5 [14–76] to 6.5 [0–45], median [range], P = 0.0002) and GPi stimulation (from 34 [7–63] to 0 [0–22], P = 0.00003) at the last follow‐up, with mean improvements of 60.73% and 93.78%, respectively, detailed in Table 2. Several patients were completely alleviated of dyskinesia, as described for one case (Video S1). Also, relative to the patients in STN group (60.73 ± 40.29%), GPi patients showed a greater reduction in LID as reflected by their UDysRS scores (93.78 ± 14.15%, P = 0.0003), and shown in Figure 1A and B.
Table 2.
Effect of STN and GPi deep brain stimulation on UDysRS sub‐score at the last follow‐up.
| UDysRS | Max value (scores) | Preoperative/last follow‐up | Improvement change, % | P value | ||
|---|---|---|---|---|---|---|
| STN (n = 20) | GPi (n = 23) | STN (n = 20) | GPi (n = 23) | |||
| Total score | 104 | 27.5 (14–76)/6.5 (0–45) | 34 (7–63)/0 (0–22) | 60.73 ± 40.29 | 93.78 ± 14.15 | 0.0003* |
|
Part 1 on‐dyskinesia (item 1–11) |
44 | 13 (7–36)/5 (0–23) | 15 (3–27)/0 (0–10) | 55.83 ± 40.88 | 94.59 ± 14.28 | 0.0001* |
|
Part 2 off‐dystonia (item 12–15) |
16 | 2 (0–16)/0 (0–4) | 0 (0–13)/0 (0–0) | 97.50 ± 7.91 | 100 | 0.371 |
|
Part 3 impairment (item 16–22) |
28 | 7.5 (2–20)/1.5 (0–15) | 12 (2–18)/0 (0–9) | 57.34 ± 50.75 | 92.78 ± 16.34 | 0.001* |
| Face | 4 | 0 (0–3)/0 (0–3) | 0 (0–3)/0 (0–0) | 81.25 ± 37.20 | 100 | 0.104 |
| Neck and trunk | 8 | 4 (0–6)/0.5 (0–6) | 4 (0–6)/0 (0–3) | 62.98 ± 46.20 | 92.92 ± 18.98 | 0.010* |
| Arms and legs | 16 | 4 (0–12)/1 (0–9) | 7 (2–12)/0 (0–6) | 75.00 (−150 to 100) | 91.44 ± 17.45 | 0.004* |
|
Part 4 disability (item 16–22) |
16 | 4 (1–15)/1 (0–7) | 4 (2–11)/0 (0–8) | 57.25 ± 47.50 | 86.01 ± 43.63 | 0.007* |
Values are presented as mean ± SD or median (range). GPi, globus pallidus interna; STN, subthalamic nucleus; UDysRS, the Unified Dyskinesia Rating Scale.
P < 0.05.
Figure 1.
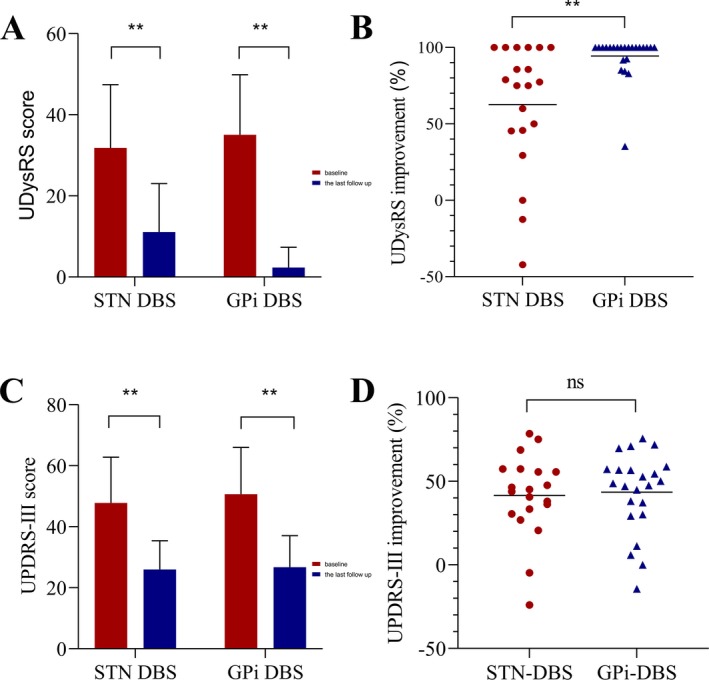
The effect of STN‐DBS and GPi‐DBS on the UDysRS scores and drug‐off UPDRS‐Ⅲ score in 43 patients with LID. (A) UDysRS scores of patients with STN‐DBS and GPi‐DBS at baseline and the last follow‐up. (B) The comparison of efficacy for clinical improvement of UDysRS between STN‐DBS and GPi‐DBS. (C) Drug‐off UPDRS‐Ⅲ scores of patients with STN‐DBS and GPi‐DBS at baseline and the last follow‐up. (D) The comparison of efficacy for the clinical improvement of drug‐off UPDRS‐Ⅲ between STN‐DBS and GPi‐DBS. "**" indicates P < 0.01. ns, non‐significant; DBS, deep brain stimulation; GPi, globus pallidus interna; STN, subthalamic nucleus; UDysRS, Unified Dyskinesia Rating Scale; UPDRS‐Ⅲ, Unified Parkinson Disease Rating Scale part Ⅲ.
At the last follow‐up, 85% (17/20) of patients with STN‐DBS had an improvement in dyskinesia and 70% (14/20) of the patients reported at least 50% improvement. However, three patients continued to experience persistent dyskinesia, and even two of these patients showed deteriorating dyskinesia. Interestingly, two patients with refractory or incomplete benefit in dyskinesia after STN‐DBS, despite multiple programming attempts noted marked benefit with almost complete resolution of dyskinesia after GPi‐DBS.
All the patients in the GPi group obtained excellent relief from dyskinesia. Among them, 78.26% (18/23) of the patients displayed at least 90% improvement, 17.39% (4/23) of patients showed medium improvement of dyskinesia (50–90% improvement), and only one patient noted a clinical improvement of <50%.
Outcomes of subitems of UDysRS scale
We also analyzed the effect of GPi stimulation and STN stimulation on different sub‐items of the UDysRS scale. As shown in Table 2, GPi‐DBS showed superior clinical improvement over STN‐DBS in on‐dyskinesia (P = 0.0001), impairment (P = 0.001) and disability (P = 0.007), except for off‐dystonia (P > 0.05). Moreover, significant differences of LID improvement between STN and GPi were observed in the neck and trunk (P = 0.010) and the arms and legs (P = 0.004). However, significantly better effects of GPi were not found with respect to face dyskinesia (P = 0.104).
Secondary outcomes
Outcome of drug‐off UPDRS‐Ⅲ
At the last follow‐up, mean improvement of drug‐off UPDRS‐Ⅲ was 41.50% (from 47.85 ± 14.95 to 26.00 ± 9.44, P = 0.0002) and 43.56% (from 50.68 ± 15.36 to 26.75 ± 10.35, P = 0.00005), respectively for STN and GPi‐DBS, as described in Figure 1C. However, there was no significant difference between STN and the GPi‐DBS for drug‐off UPDRS‐III (P = 0.609), detailed in Figure 1D.
Outcome of LED
As shown in Figure 2A, compared with baseline, at the last follow‐up, LED was significantly decreased in patients with STN (from 827.04 ± 376.39 mg to 414.50 ± 291.95 mg, P = 0.0001) and GPi‐DBS (from 981.76 ± 426.09 mg to 760.78 ± 344.42 mg, P = 0.012), and relative to GPi‐DBS, patients with STN‐DBS showed a greater LED reduction (43.81% vs. 13.29%, P = 0.021), as displayed in Figure 2B. In the STN group, 95% (19/20) of the patients obtained LED reduction. One patient stopped all dopaminergic medications. Comparatively, the reduction in medication was smaller for GPi‐treated patients, 65.22% (15/23) of patients noted LED reduction, and six patients increased medication dose for optimal symptom control.
Figure 2.
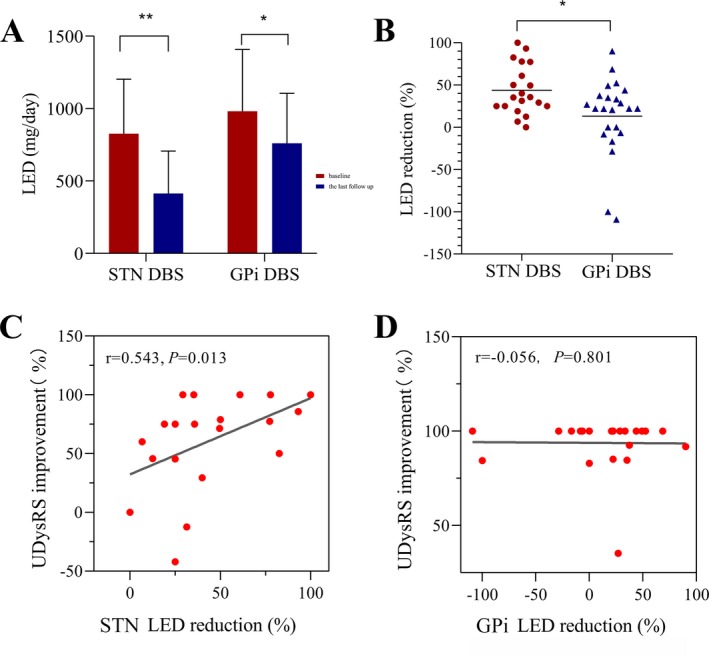
The effect of STN‐DBS and GPi‐DBS on LED in 43 patients with LID and the correlation analysis between LED reduction and UDysRS improvement. (A) LED of patients with STN‐DBS and GPi‐DBS at baseline and the last follow‐up. (B) The comparison of LED reduction between STN‐DBS and GPi‐DBS. Spearman's correlation analysis demonstrated significant relationships between changes of UDysRS score and LED reduction in STN group (C), while not in GPi group (D). "**" indicates P < 0.01, "*" indicates P < 0.05. DBS, deep brain stimulation; GPi, globus pallidus interna; STN, subthalamic nucleus; LED, levodopa equivalent dose; UDysRS, Unified Dyskinesia Rating Scale.
Correlation analysis
None of the baseline factors predicted LID clinical improvements (all P > 0.05) in both STN and GPi groups. Drug‐off UPDRS‐Ⅲ score changes were not significantly associated with UDysRS score variations (P = 0.256 and 0.262, respectively for STN and GPi‐DBS). However, LED reduction was significantly associated with UDysRS changes in the STN group (r = 0.543, P = 0.013, Figure 2C), while no significant correlations were detected in the GPi group (r = −0.056, P = 0.801, Figure 2D).
Programming settings
DBS programing settings are summarized in Tables S3 and S4. The stimulation mode included monopolar, double monopolar, and interleaving mode. The mean amplitude, frequency and pulse width were 2.63 ± 0.49 V vs. 2.77 ± 0.46 V (P = 0.102), 146.67 ± 14.43 Hz vs. 148.74 ± 14.74 Hz (P = 0.138) and 73.10 ± 10.93 μs vs. 79.78 ± 12.38 μs (P = 0.009), respectively for STN and GPi‐DBS. Different programming settings were tested during follow‐up visits and the dorsal contacts in the STN group were consistently utilized due to potential suppression of dyskinesia.
Adverse effects
A total of 19 adverse events occurred in 11 STN patients and in five GPi patients. Half (10/20) of patients in the STN‐DBS group reported stimulation‐induced dyskinesia (SID) after the surgery, which was completely or partially relieved by changing to upper contact stimulation, lower amplitude stimulation, or bipolar stimulation. On the other hand, in the GPi group, two patients also experienced SID, which was rescued by changing to lower contact stimulation. Furthermore, two patients reported increasing bradykinesia during programming sessions with GPi‐DBS (none reported in the STN group). Changing to upper contact stimulation resolved the increasing bradykinesia in one patient. One lead fracture and 1 repeated infection of the incision occurred in two STN patients. One lead fracture and 1 toe tic were observed in patients with GPi‐DBS. Hallucination was noted in one patient with STN‐DBS.
Discussion
DBS surgery is an established treatment which can effectively modulate the motor symptoms in PD. In the current study, we evaluated and compared the efficacy for management of dyskinesia in and between STN‐DBS and GPi‐DBS and we also explored the underlying mechanisms. We observed that both STN and GPi‐DBS can not only improve the drug‐off UPDRS‐Ⅲ scores but also relieve LID symptoms. After stimulation for 18 (6–30) months, UDysRS scores were significantly reduced from baseline in both groups, with a greater reduction in patients receiving GPi‐DBS (GPi vs. STN, 93.78 ± 14.15% vs. 60.73 ± 40.29%, P = 0.0003). As to the underlying mechanism, both groups showed a decrease in LED, but the STN group had a greater reduction (GPi vs. STN, 13.29% vs. 43.81%, P = 0.021). Furthermore, LID clinical outcome was significantly correlated with LED reduction in the STN group (r = 0.543, P = 0.013), but not in GPi‐DBS (r = −0.056, P = 0.801). In the STN‐DBS group, LID is likely primarily modulated through a reduction in dopaminergic medication while GPi stimulation may provide a direct anti‐dyskinesia effect. To our knowledge, this current single‐center cohort study has one of the largest sample sizes and longest follow periods to compare the efficacy of STN and GPi‐DBS for pre‐operative LID.
LID can be difficult to manage in some PD patients with few medical options or strategies, some of which can lead to deteriorating parkinsonism and motor fluctuations. DBS is an excellent alternative for refractory patients. In our cohort, we noted that both GPi (93.78 ± 14.15%) and STN (60.73 ± 40.29%) targets are effective in controlling LID, with the GPi target showing better efficacy than STN (P = 0.0003). The current results are in line with previous studies, which have shown a reduction in dyskinesia with stimulation of the GPi and STN (89% vs. 62%).25 A review study also showed a 20–83% and 47–88% improvement in LID, respectively for STN and GPi‐DBS.29 A meta‐analysis has also indicated that GPi‐DBS offered a more robust reduction in dyskinesia than STN‐DBS.12 However, compared to previous studies, the present study has a large sample size and a longer follow‐up time, and focused particularly on preoperative LID.
As for the four subscales of UDysRS, a comparison of results between STN and GPi showed significant differences in on‐dyskinesia (P = 0.0001), impairment (P = 0.001) and disability (P = 0.007), which primarily measured the symptom of peak‐dose dyskinesia. However, no significant differences were seen in the off‐dystonia subscale between the two targets. The advantage of the GPi target may be specific for LID, and not for typical PD symptoms (i.e., tremor, rigidity, and bradykinesia), as Follett et al. also indicated that no significant differences were reported in a large randomized controlled PD trial (http://ClinicalTrials.gov numbers, NCT00056563 and NCT01076452) between GPi and STN.18, 30 The lack of significant differences in face dyskinesia improvement may be due to the small sample size of face dyskinesia in our study. The featured advantage of GPi for LID was highlighted in the current study, and we provide new evidence.
In line with previous studies, the anti‐dyskinesia effect of STN was mostly dependent on drug reduction,24 as 95% (19/20) of the patients had a LED reduction. Furthermore, our study showed a significant correlation (r = 0.543, P = 0.013) between LED reduction and dyskinesia improvement, indicating that dyskinesia suppression can be predicted by decreases in LED. Previous studies have also indicated that levodopa‐drugs reduction can reverse LID and as such may be pathogenically involved in LID progression.19, 24 STN‐DBS resulted in a LED reduction for LID and control of the typical PD symptoms, which are believed to be underpinned by a stable and continuous functional state with reduced fluctuations in basal ganglia activity. STN‐DBS also mimicked the effect of continuous dopamine stimulation, thereby decreasing the risk for dyskinesia.24, 31
The primary disadvantage of STN‐DBS for dyskinesia is the indirect mechanism of action on these symptoms: dyskinesias improve only if medications can be withdrawn or reduced. In certain patients, programming might be difficult due to suboptimal lead location or specific clinical presentation. If LED reduction post‐operatively is not attained, dyskinesia may continue.8, 29 In addition, this treatment is sometimes associated with persistent dyskinesias, which may be a side effect of stimulation, even with a reduction in medication.8 Two of our patients suffered deterioration of dyskinesia even after LED reduction, one of these patients has been switched to GPi‐DBS with good response.
A direct anti‐dyskinesia effect of STN‐DBS was also noticed in our study. It was found in 10 patients who experienced SID, which were rescued through changing the stimulation to the uppermost contact. As reported in some studies, the direct anti‐dyskinesia effect is induced by stimulation of the area above the STN rather than the STN itself.15, 32, 33 In the area above the STN, pallidothalamic, pallidosubthalamic, and subthalamopallidal fibers are densely distributed. It appears that stimulation of these fibers may cause effects similar to thalamic or pallidal DBS and therefore inhibit peak‐dose dyskinesia.31, 34
As for GPi‐DBS, the direct anti‐dyskinesia effect was the primary effect. LED reduction was only 13.29%, and six patients have increased LED without worsening of LID. No significant association was found between LED reduction and LID improvement for GPi‐DBS. Direct treatment of dyskinesias with DBS of the GPi can widen the therapeutic window for dopaminergic medications and facilitate a much higher dosage,35 which has been supported in our study by a subset of six patients. We suspect that patients in the GPi group do not require marked medications adjustments in follow‐up as they lack to report side effects, namely dyskinesia or wearing off. Although not consistently studied, it is possible that GPi is not as efficient in management of other non‐motor symptoms requiring higher doses of dopaminergic medications.
To date, there is little understanding of the exact mechanism of LID and the direct anti‐dyskinesia effect of GPi.36 An intact pallido‐thalamic tract is a prerequisite for the development of LID.13, 34 LID may be correlated with an abnormal pattern of the neuronal activity within the GPi.37 Dyskinesia might also arise from an abnormal balance of activity within different functional zones of the nucleus (ventral part vs. dorsal part of the GPi) and GPi stimulation may suppress this abnormal activity.8, 29
In the GPi group, there were two patients who experienced SID, and SID was reversed by stimulation of the lower contact. Some studies indicated that stimulating the ventral part of the nucleus can exert an anti‐dyskinesia effect.11, 38 In addition, bradykinesia in GPi‐DBS should also not be ignored. This has been reported in many studies and may seriously affect the effectiveness of the treatment.39 Furthermore, GPi‐DBS may consume more power.
Overall, considering that LED reduction may induce much unexpected levodopa‐dependent clinical, non‐motor symptoms such as psychiatric problems or gastrointestinal problems, GPi might be the optimal target for patients suffering from LID through a direct anti‐dyskinesia effect. Those with pre‐existing psychiatric, cognitive conditions, prominent gait disorder, axial symptoms, or those prone to falling might also be suitable for GPi‐DBS.17, 22, 23, 40, 41, 42 If a reduction in medication is the desired goal of surgery or the patient was affected primarily by medication side effects other than dyskinesia, DBS of the STN may be the preferable approach. Additionally, STN‐DBS is commonly considered and recommended for patients with severe bradykinesia, akinesia, off times and fluctuations before surgery.25 Thus, selection of the target should be specific to the patient. Additionally, the programming procedure is also important, the programming settings, such as, the choice of contact, the voltage, the pulse width, and the frequency all could influence the eventual dyskinesia suppression effect.15, 43
Some limitations must be considered in our study. First, our study was a retrospective study. A randomized controlled trial should be performed in the future. Second, the treatment of PD and LID patients with DBS, was a comprehensive treatment, and therefore we should take into account different aspects of these conditions in future studies, such as cognitive evaluation, psychiatric problems and other non‐motor symptoms. In the current study subjects were excluded during the screening phase if they had serious cognitive or psychiatric problems which could affect the study.
In conclusion, compared to the STN target, GPi‐DBS showed a better clinical outcome for preoperative LID (P = 0.0003) through a direct anti‐dyskinesia effect. STN‐DBS mainly controlled LID by way of LED reduction, which might be a predictive factor for LID outcome in STN patients (r = 0.543, P = 0.013). The final treatment strategy should be determined by patient specific features of LID with comprehensive consideration of the condition.
Author Contributions
Study concept and design: Adolfo Ramirez‐Zamora and Fan‐Gang Meng. Data collection: Shi‐ying Fan, Qiao Wang, Shimabukuro Michitomo, Chun‐lei Han. Analysis and interpretation: Kai‐Liang Wang, Shi‐ying Fan, Wei Hu, Robert S. Eisinger, Alexander Han, Feng Wang. Drafting of the manuscript: Kai‐Liang Wang, Shi‐ying Fan. Critical revision of the manuscript: Adolfo Ramirez‐Zamora, Fan‐Gang Meng, Wei Hu. Study supervision: Fan‐Gang Meng, Adolfo Ramirez‐Zamora, Jian‐Guo Zhang.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Supporting information
Table S1. Target coordinates in STN group.
Table S2. Target coordinates in GPi group.
Table S3. Programming settings for STN‐DBS.
Table S4. Programming settings for GPi‐DBS.
Video S1. Video of one patient with complete dyskinesia improvement after DBS. This patient is a chinese women, 66 years old. She experienced the first emergence of fatigue at 55 years old, and a diagnosis of Parkinson’s disease was established at the same year. Her main complaints were tremor, stiffness, fatigue, and bradykinesia. After 11 years of drug therapy, she suffered from severe levodopa‐induced dyskinesia and fluctuations. Then she underwent DBS surgery in our center. The operation was successful and she got significant improvement of her parkinson’s symptoms (drug‐off UPDRS‐Ⅲ, from 55 to 23.8, 56.73% improvement) and complete control of dyskinesia (UDysRS, from 63 to 0, 100% improvement) after surgery, which has persisted for 2 years.
Acknowledgments
We thank the patients and families for participating in this study. We thank the medical ethics committee of IRB of Beijing Tiantan Hospital Affiliated to Capital Medical University for the approval of our study. This study was funded by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201820), The Key Research Project of Ningxia (2018BFG02007) and National Natural Science Foundation of China (81971070). The funder had no role in the study design, data collection, or analysis, the decision to publish or the preparation of the manuscript.
Shi‐Ying Fan and Kai‐Liang Wang contribute to the first author equally.
Fan‐Gang Meng and Adolfo Ramirez‐Zamora contribute to the corresponding author equally.
Funding Information
This study was funded by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201820), The Key Research Project of Ningxia (2018BFG02007) and National Natural Science Foundation of China (81971070).
Funding Statement
This work was funded by The Key Research Project of Ningxia grant 2018BFG02007; National Natural Science Foundation of China grant 81971070; Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support grant ZYLX201820.
Contributor Information
Adolfo Ramirez‐Zamora, Email: adolfo.ramirez-zamora@neurology.ufl.edu.
Fan‐Gang Meng, Email: mengfg@ccmu.edu.cn.
References
- 1. Kalia LV, Lang AE. Parkinson's disease. The Lancet 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- 2. Dragašević‐Mišković N, Petrović I, Stanković I, Kostić VS. Chemical management of levodopa‐induced dyskinesia in Parkinson’s disease patients. Expert Opin Pharmacother 2019;20:219–230. [DOI] [PubMed] [Google Scholar]
- 3. Vijayakumar D, Jankovic J. Drug‐induced dyskinesia, part 1: treatment of levodopa‐induced dyskinesia. Drugs 2016;76:759–777. [DOI] [PubMed] [Google Scholar]
- 4. Sharma S, Singh S, Sharma V, et al. Neurobiology of l‐DOPA induced dyskinesia and the novel therapeutic strategies. Biomed Pharmacother 2015;70:283–293. [DOI] [PubMed] [Google Scholar]
- 5. Turcano P, Mielke MM, Bower JH, et al. Levodopa‐induced dyskinesia in Parkinson disease: a population‐based cohort study. Neurology 2018;91:e2238–e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin MM, Laureno R. Less pulsatile levodopa therapy (6 doses daily) is associated with a reduced incidence of dyskinesia. J Mov Disord 2019;12:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhuri KR, Jenner P, Antonini A. Should there be less emphasis on levodopa‐induced dyskinesia in Parkinson’s disease? Mov Disord 2019;34:816–819. [DOI] [PubMed] [Google Scholar]
- 8. Follett KA. Comparison of pallidal and subthalamic deep brain stimulation for the treatment of levodopa‐induced dyskinesias. Neurosurg Focus 2004;17:E3. [DOI] [PubMed] [Google Scholar]
- 9. Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo‐controlled trial of ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord 2017;32:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips JR, Eissa AM, Hewedi DH, et al. Neural substrates and potential treatments for levodopa‐induced dyskinesias in Parkinson’s disease. Rev Neurosci 2016;27:729–738. [DOI] [PubMed] [Google Scholar]
- 11. Peppea A, Pierantozzi M, Altibrandic MG, et al. Bilateral GPi DBS is useful to reduce abnormal involuntary movements in advanced Parkinson's disease patients, but its action is related to modality and site of stimulation. Eur J Neurol 2001;8:579–586. [DOI] [PubMed] [Google Scholar]
- 12. Liu Y, Li F, Luo H, et al. Improvement of deep brain stimulation in dyskinesia in Parkinson's disease: a meta‐analysis. Front Neurol 2019;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sankar T, Lozano AM. Surgical approach to l‐dopa‐induced dyskinesias. Int Rev Neurobiol 2011;98:151–171. [DOI] [PubMed] [Google Scholar]
- 14. Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 2013;12:37–44. [DOI] [PubMed] [Google Scholar]
- 15. Oyama G, Foote KD, Jacobson CE 4th, et al. GPi and STN deep brain stimulation can suppress dyskinesia in Parkinson's disease. Parkinsonism Relat Disord 2012;18:814–818. [DOI] [PubMed] [Google Scholar]
- 16. Chiou SM, Lin YC, Huang HM. One‐year outcome of bilateral subthalamic stimulation in Parkinson disease: an eastern experience. World Neurosurg 2015;84:1294–1298. [DOI] [PubMed] [Google Scholar]
- 17. Bonenfant J, Drapier S, Houvenaghel JF, et al. Pallidal stimulation in Parkinson's patients with contraindications to subthalamic target: a 3 years follow‐up. Parkinsonism Relat Disord 2017;34:20–25. [DOI] [PubMed] [Google Scholar]
- 18. Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology 2012;79:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonin C, Tir M, Devos D, et al. Reduced levodopa‐induced complications after 5 years of subthalamic stimulation in Parkinson's disease: a second honeymoon. J Neurol 2009;256:1736–1741. [DOI] [PubMed] [Google Scholar]
- 20. Volkmann J, Albanese A, Kulisevsky J, et al. Long‐term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson's disease. Mov Disord 2009;24:1154–1161. [DOI] [PubMed] [Google Scholar]
- 21. Volkmann J, Allert N, Voges J, et al. Long‐term results ofbilateral pallidal stimulation in Parkinson’s disease. Ann Neurol 2004;55:871–875. [DOI] [PubMed] [Google Scholar]
- 22. Ramirez‐Zamora A, Ostrem JL. Globus pallidus interna or subthalamic nucleus deep brain stimulation for Parkinson disease: a review. JAMA Neurol 2018;75:367–372. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow‐up. Brain 2005;128:2240–2249. [DOI] [PubMed] [Google Scholar]
- 24. Russmann H, Ghika J, Combrement P, et al. L‐Dopa‐induced dyskinesia improvement after STN‐DBS depends upon medication reduction. Neurology 2004;63:153–155. [DOI] [PubMed] [Google Scholar]
- 25. Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 2005;62:554–560. [DOI] [PubMed] [Google Scholar]
- 26. Berg D, Lang AE, Postuma RB, et al. Changing the research criteria for the diagnosis of Parkinson's disease: obstacles and opportunities. Lancet Neurol 2013;12:514–524. [DOI] [PubMed] [Google Scholar]
- 27. Goetz CG, Nutt JG, Stebbins GT. The Unified Dyskinesia Rating Scale: presentation and clinimetric profile. Mov Disord 2008;23:2398–2403. [DOI] [PubMed] [Google Scholar]
- 28. Juhász A, Deli G, Aschermann Z, et al. How efficient is subthalamic deep brain stimulation in reducing dyskinesia in Parkinson's disease? Eur Neurol 2017;77:281–287. [DOI] [PubMed] [Google Scholar]
- 29. Munhoz RP, Cerasa A, Okun MS. Surgical treatment of dyskinesia in Parkinson's disease. Front Neurol 2014;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep‐brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- 31. Kim JH, Chang WS, Jung HH, Chang JW. Effect of subthalamic deep brain stimulation on levodopa‐induced dyskinesia in Parkinson's disease. Yonsei Med J 2015;56:1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishikawa Y, Kobayashi K, Oshima H, et al. Direct relief of levodopa‐induced dyskinesia by stimulation in the area above the subthalamic nucleus in a patient with Parkinson's disease. Neurol Med Chir (Tokyo) 2010;50:257–259. [DOI] [PubMed] [Google Scholar]
- 33. Alterman RL, Shils JL, Gudesblatt M, Tagliati M. Immediate and sustained relief of levodopa‐induced dyskinesias after dorsal relocation of a deep brain stimulation lead. Neurosurg Focus 2004;17:E6. [DOI] [PubMed] [Google Scholar]
- 34. Katayama Y, Oshima H, Kano T, et al. Direct effect of subthalamic nucleus stimulation on levodopa‐induced peak‐dose dyskinesia in patients with Parkinson's disease. Stereotact Funct Neurosurg 2006;84:176–179. [DOI] [PubMed] [Google Scholar]
- 35. Stanzione P, Mazzone P, Peppe A, et al. Antiparkinsonian and anti‐levodopa‐induced dyskinesia effects obtained by stimulating the same site within the GPi in PD. Neurology 1998;51:1776–1777. [DOI] [PubMed] [Google Scholar]
- 36. Gour J, Edwards R, Lemieux S, et al. Movement patterns of peak‐dose levodopa‐induced dyskinesias in patients with Parkinson's disease. Brain Res Bull 2007;74:66–74. [DOI] [PubMed] [Google Scholar]
- 37. Li X, Zhuang P, Li Y. Altered neuronal firing pattern of the basal ganglia nucleus plays a role in levodopa‐induced dyskinesia in patients with Parkinson's disease. Front Hum Neurosci 2015;9:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Angeli A, Akram H, Zacharia A, et al. Varying time‐course of effects of high frequency stimulation of sub‐regions of the globus pallidus in patients with parkinson's disease. Parkinsonism Relat Disord 2015;21:597–602. [DOI] [PubMed] [Google Scholar]
- 39. Berman BD, Starr PA, Marks WJ Jr, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial‐cervical dystonia. Stereotact Funct Neurosurg 2009;87:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rouaud T, Dondaine T, Drapier S, et al. Pallidal stimulation in advanced Parkinson's patients with contraindications for subthalamic stimulation. Mov Disord 2010;25:1839–1846. [DOI] [PubMed] [Google Scholar]
- 41. Odekerken VJ, Boel JA, Geurtsen GJ, et al. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology 2015;84:1355–1361. [DOI] [PubMed] [Google Scholar]
- 42. St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta‐regression of the long‐term effects of deep brain stimulation on balance and gait in PD. Neurology 2010;75:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Merola A, Zibetti M, Artusi CA, et al. 80 Hz versus 130 Hz subthalamic nucleus deep brain stimulation: effects on involuntary movements. Parkinsonism Relat Disord 2013;19:453–456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Target coordinates in STN group.
Table S2. Target coordinates in GPi group.
Table S3. Programming settings for STN‐DBS.
Table S4. Programming settings for GPi‐DBS.
Video S1. Video of one patient with complete dyskinesia improvement after DBS. This patient is a chinese women, 66 years old. She experienced the first emergence of fatigue at 55 years old, and a diagnosis of Parkinson’s disease was established at the same year. Her main complaints were tremor, stiffness, fatigue, and bradykinesia. After 11 years of drug therapy, she suffered from severe levodopa‐induced dyskinesia and fluctuations. Then she underwent DBS surgery in our center. The operation was successful and she got significant improvement of her parkinson’s symptoms (drug‐off UPDRS‐Ⅲ, from 55 to 23.8, 56.73% improvement) and complete control of dyskinesia (UDysRS, from 63 to 0, 100% improvement) after surgery, which has persisted for 2 years.


