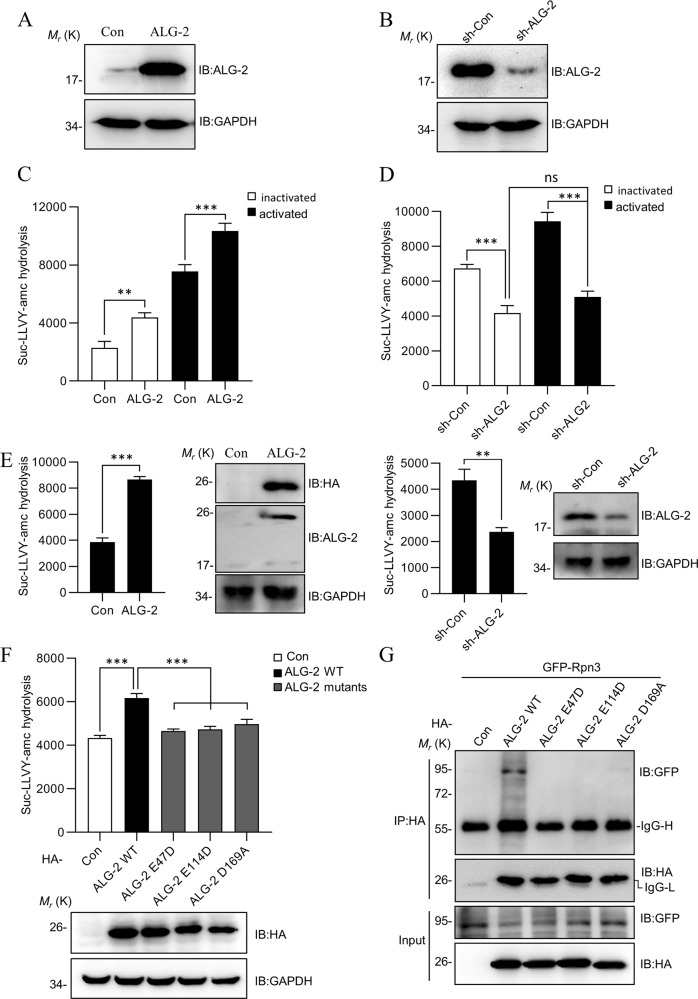
Fig. 3. ALG-2 enhances the activity of the proteasome.
a, b The expression levels of ALG-2 in ALG-2-OX and ALG-2-KD Jurkat cell lines. The protein levels of ALG-2 and GAPDH are determined by western blotting. c, d ALG-2 enhances the activity of proteasome in Jurkat cells. The ALG-2-OX and ALG-2-KD Jurkat cells, either inactivated or activated, were incubated in digitonin permeabilization buffer with slight sonication. Cell lysates were incubated for 1 h at 37 °C in reaction buffer with Suc-LLVY-AMC. The activity of proteasome was quantitated by AMC fluorescence intensity in a microtiter plate reader at λEX = 380 nm/λEM = 460 nm. e ALG-2 enhances the activity of proteasome in stimulated PBMCs. PBMCs were infected with ALG-2 retrovirus (left panel) or sh-ALG-2 retrovirus (right panel) for 40 h and activated by PMA and ionomycin for 10 h. Then the cells were measured the activity of proteasome as c. f ALG-2 enhances the activity of proteasome dependent on its calcium-binding sites. ALG-2-KD Jurkat cells were infected with ALG-2 WT, ALG-2 E47D, ALG-2 E114D, and ALG-2 D169A retrovirus for 48 h, then activated by PMA and ionomycin for 10 h. Proteasomal activities were assessed by Suc-LLVY-AMC cleavage as c. g Co-immunoprecipitation assays of ALG-2 mutants with Rpn3. Data shown were representative of three independent experiments. Error bars indicate SD. **P < 0.01; ***P < 0.001.