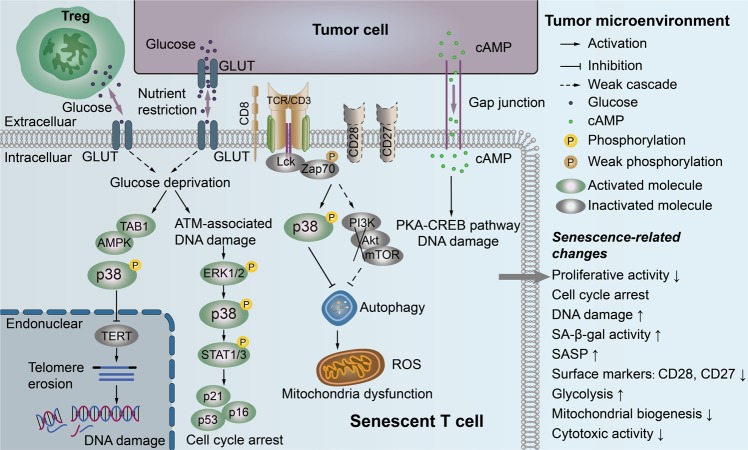
Fig. 2. Molecular pathways involved in T-cell senescence in the tumor microenvironment.
MAPK p38 plays a central role in the regulatory network of T-cell senescence. The glucose deprivation induced by tumor cells and/or Treg cells is attributed to the activation of AMPK-TAB1-dependent p38 activation, which induces TERT downregulation and/or telomere erosion, as well as the DNA damage response. Furthermore, the competition for glucose also triggers the ATM-associated DNA damage response and activates the MAPK ERK1/2 and p38 signaling pathways in T cells that functionally cooperate with the transcription factors STAT1 and STAT3 to induce cell cycle arrest and cell senescence in effector T cells. Tumor- and Treg cell-induced downregulation of CD28 and TCR signaling can activate MAPK p38 signaling or/and inhibit the PI3K-Akt-mTOR axis, subsequently inactivating autophagy and inducing mitochondrial dysfunction and elevated ROS levels in senescent T cells. Tumor-derived cAMP can be directly transferred into target T cells through gap junctions to activate PKA-CREB signaling, resulting in the activation of DNA damage response and induction of senescence in T cells. MAPK mitogen-activated protein kinase, TAB1 TAK1-binding protein 1, AMPK AMP-responsive protein kinase, ATM ataxia telangiectasia mutated, ERK extracellular signal-regulated protein kinase, STAT signal transducer and activator of transcription, cAMP endogenous cyclic adenosine monophosphate, Treg regulatory T cell, TERT telomerase reverse transcriptase, PKA protein kinase A, CREB cAMP response element-binding protein, SASP senescence-associated secretory phenotype, ROS reactive oxygen species.