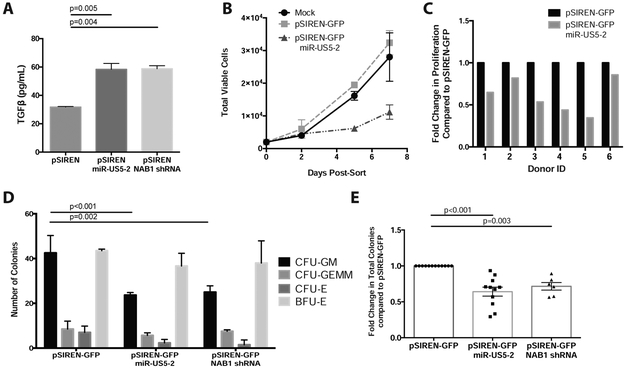
Figure 3. HCMV miR-US5-2 directly targets NAB1 to upregulate TGF-β in HPCs and mediate myelosuppression.
A) Kasumi-3 cells were transfected with pSIREN-GFP plasmids expressing miR-US5-2 or an shRNA against NAB1 and cultured for 24hrs. Pure populations of viable, transfected (GFP+) cells were isolated by FACS and recovered overnight. Supernatants were analyzed by TGF-β ELISA following an additional 24hrs in serum-free media. B and C) CD34+ HPCs were transfected as in A for 48hrs. Pure populations of viable, transfected (GFP+) cells were isolated by FACS and plated in SFEMII to support progenitor cell proliferation for 7 days. Total viable cells were manually counted at 2, 5 and 7 days post plating. The average proliferation for one representative experiment is shown in B with error bars representing standard deviation for replicate wells. Primary CD34+ HPCs from 6 independent donors were transfected with pSIREN-GFP-miR-US5-2 and the fold change in proliferation compared to control vector (pSIREN-GFP) shown in C. Two way ANOVA was performed comparing all donors in C, p<0.05. D and E) CD34+ HPCs were transfected and isolated as above. Pure populations were plated at 500 cells per dish in Methocult H4434 in triplicate for 14 days. Specific myeloid colony types are shown from one representative experiment in D. The average total myeloid colonies from independent CD34+ HPC donors are shown in E for miR-US5-2 (N=11) and NAB1 shRNA (N=5). Error bars represent standard deviation for replicate wells (D) or SEM for replicate experiments (E), significance determined by two-way (D) or one-way (E) ANOVA with Tukey post-test.