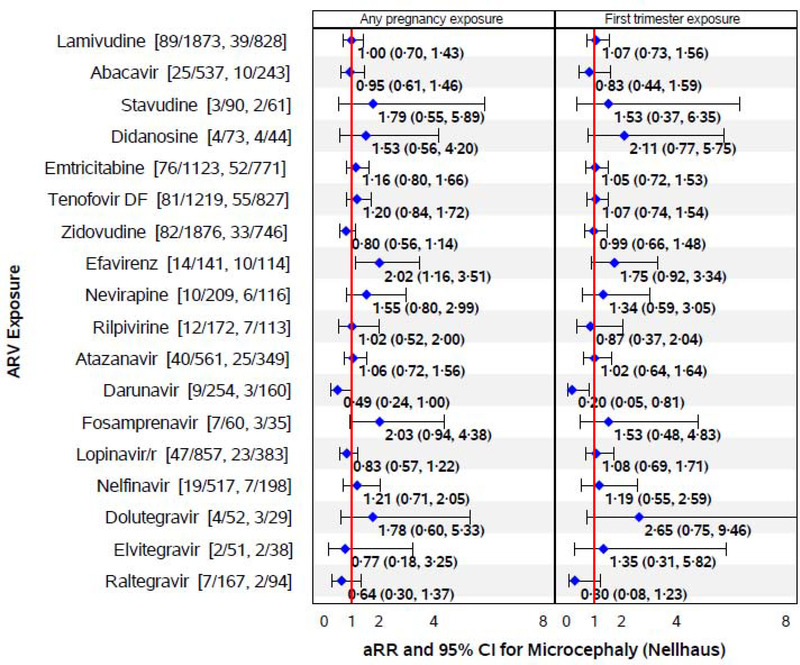
Figure 1. Associations of in utero ARV Exposures with Microcephaly by Nellhaus criteria.
Numbers in brackets after each drug name indicate number of microcephaly cases among those exposed to the specific ARV drug during pregnancy, and number of cases among those exposed to that ARV drug during the first trimester. Adjusted relative risks (aRRs) are based on modified Poisson regression models adjusting for low education, low household income, alcohol use during pregnancy, and birth cohort (2007–2010, 2011–2014 and 2015–2017 vs <2007). Models based on 1976 children born after 2007 for darunavir, raltegravir, fosamprenavir, and IIs (as drug class), 927 children born after 2011 for rilpivirine, and 506 children born after 2013 for dolutegravir and elvitegravir; all other ARV drugs are evaluated in the full SMARTT cohort (N=2983).