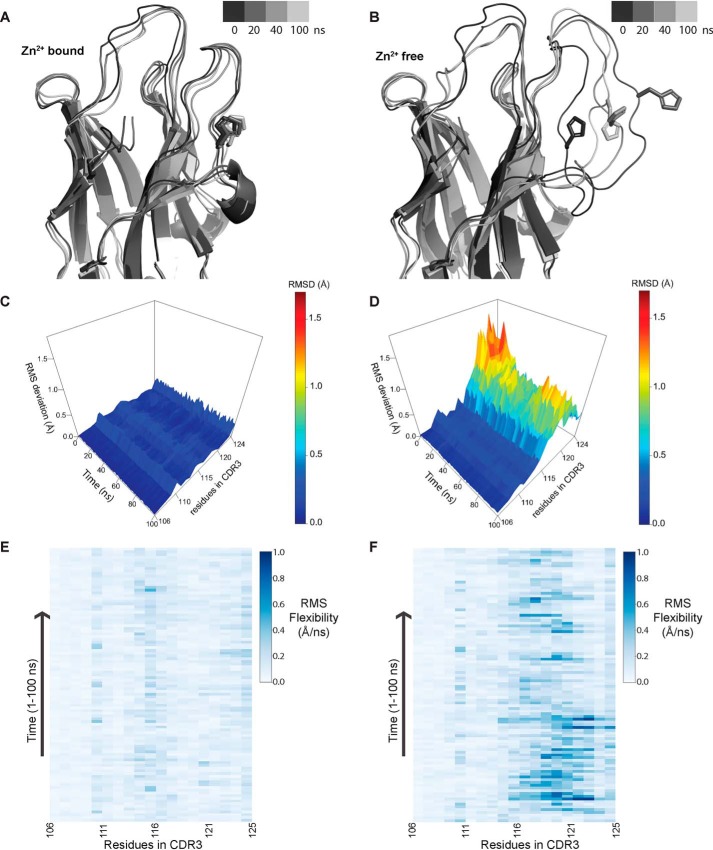
Figure 5.
Molecular dynamics simulations highlighting the role of Zn2+ in providing conformational stability to CDR3. A and B, representation of structure along the trajectory at different time points in the Zn2+-bound (A) and Zn2+-free (B) state. In the presence of Zn2+, CDR3 loop conformation is restrained, whereas in the absence of Zn2+, it becomes more mobile, which allows the His residue (involved in coordination to Zn2+) to flip outside. C and D, RMSD of residues in the CDR3 region over the course of trajectory in the presence of Zn2+ (C) and in the absence of Zn2+ (D). E and F, RMS flexibility of residues in the CDR3 region in Å/ns in the presence of Zn2+ (E) and in the absence of Zn2+ (F); only side-chain atoms were taken for calculation. Conformational flexibility of residues from 115 to 121 significantly increases in the absence of Zn2+ coordination.