Abstract
Although autophagy is a well-known and extensively described cell pathway, numerous studies have been recently interested in studying the importance of its regulation at different molecular levels, including the translational and post-translational levels. Therefore, this review focuses on the links between autophagy and epigenetics in cancer and summarizes the. following: (i) how ATG genes are regulated by epigenetics, including DNA methylation and post-translational histone modifications; (ii) how epidrugs are able to modulate autophagy in cancer and to alter cancer-related phenotypes (proliferation, migration, invasion, tumorigenesis, etc.) and; (iii) how epigenetic enzymes can also regulate autophagy at the protein level. One noteable observation was that researchers most often reported conclusions about the regulation of the autophagy flux, following the use of epidrugs, based only on the analysis of LC3B-II form in treated cells. However, it is now widely accepted that an increase in LC3B-II form could be the consequence of an induction of the autophagy flux, as well as a block in the autophagosome-lysosome fusion. Therefore, in our review, all the published results describing a link between epidrugs and autophagy were systematically reanalyzed to determine whether autophagy flux was indeed increased, or inhibited, following the use of these potentially new interesting treatments targeting the autophagy process. Altogether, these recent data strongly support the idea that the determination of autophagy status could be crucial for future anticancer therapies. Indeed, the use of a combination of epidrugs and autophagy inhibitors could be beneficial for some cancer patients, whereas, in other cases, an increase of autophagy, which is frequently observed following the use of epidrugs, could lead to increased autophagy cell death.
Keywords: autophagy, epigenetics, cancer, DNA methylation, histone deacetylase (HDAC), histone methylation, histone methylation
1. Introduction
1.1. Basics of Autophagy
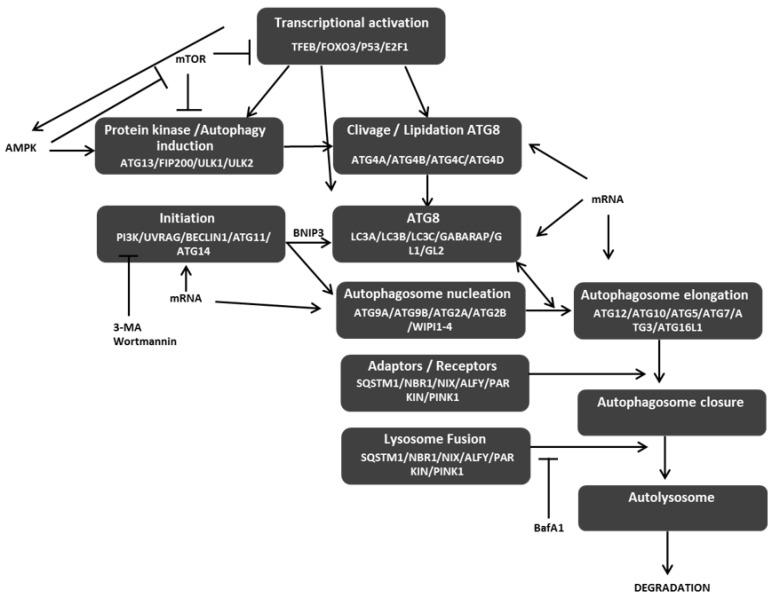
Autophagy is a multistep process involving more than 40 autophagy-related (ATG) proteins leading to the formation of a double membrane structure, called autophagosome, and the elimination of its content following its fusion with a lysosome (Figure 1) [1]. Autophagy degrades proteins or organelles, such as damaged mitochondria, and is frequently linked to cancer initiation and progression. Indeed, both pro- (e.g., resistance to starvation, chemo-resistance, etc.) and anticancer (e.g., autophagic cell death. etc.) properties have been associated to autophagy depending of the cell model, cancer grade, or cell microenvironment. Autophagy can be divided into the following four steps: (i) initiation which is controlled by the activating phosphorylation of ULK1 (Unc-51-like autophagy activating kinase 1) by AMPK while the phosphorylation of ULK1/ULK2 by mTOR (mammalian target of rapamycin kinase) leads to autophagy inhibition; (ii) formation of the phagophore which requires Beclin-1 and ATG14 (autophagy gene 14) leading to the recruitment of the PI3K kinase and the production of PI3P, shown to be essential for the phagophore initiation; (iii) elongation which is dependent of two complexes, the ATG5/ATG12/ATG16L trimeric complex which acts as an E3-like molecule and facilitates the conjugation of ATG8 proteins onto phospholipids, previously cleaved by the ATG4 proteases; and (iv) fusion involving UVRAG (ultraviolent irradiation resistance-associated), and leading to the fusion of the autophagosome with lysosomes to induce the degradation of its content by proteolytic lysosomal enzymes. Autophagy can be unselective, when the phagophore nonspecifically engulfs part of the cytoplasm, or selective, when adaptor proteins specifically link the material to degrade and the ATG8 proteins on the surface of the autophagosome to induce its selective degradation. We can cite, for example, the mitophagy, a process implying the protein NIX which specifically targets mitochondria to autophagosomes thanks to its interaction with ATG proteins.
Figure 1.
Interconnection of major autophagy steps. Autophagy induction and initiation of autophagy steps lead both to the cleavage and lipidation of autophagy gene 8 (ATG8) and autophagosome nucleation. ATG8 and protein involved in autophagosome elongation favor the closure of autophagosome and, then, its fusion with lysosome to form an autolysosome and to induce the degradation of its content. AMPK acts as an activator of autophagy, whereas mTOR acts as an inhibitor of autophagy. 3-MA and wortmannin are chemical inhibitors of early steps autophagy, whereas BafA1 blocks the fusion of autophagosomes with lysosomes. Arrows represent a positive action on the target, whereas bar-headed arrows represent an inhibition process.
1.2. Transcriptional Regulation of Autophagy
Although autophagy is a well-known and extensively described cell pathway, numerous studies have been recently interested in studying the importance of its regulation at different molecular levels, including at the translational or post-translational levels. Indeed, only a few transcriptional factors (TFs) have been described to be key regulators of the transcription of autophagy genes (for reviews see [2,3]). The helix–loop–helix transcription factor EB (TFEB) can preferentially bind to the coordinated lysosomal expression and regulation motif (CLEAR) (GTCACGTGAC) and promote the transcription of many ATG-related genes. Indeed, upon starvation, TFEB is dephosphorylated and translocated into the nucleus leading to an increase in autophagy gene transcription, and therefore autophagy. Under nutrient-rich conditions, mTOR is responsible for the phosphorylation of TFEB at the lysosomal surface, and therefore leading to its inactivation. Phosphorylation of the forkhead transcription factor FOXO3 by AKT blocks its translocation into the nucleus, therefore, inhibiting the transcription of several ATG-related genes. Another transcription factor, P53, which is frequently mutated in cancer cells, also promotes the expression of FOXO3. Moreover, P53 can also induce the expression of Sestrin proteins which activate AMPK leading to the inhibition of mTOR and the activation of autophagy. Finally, hypoxia, which could be observed in solid tumors, is associated with a decreased recruitment of NFKB and E2F1 on the BNIP3 promoter and an inhibition of the transcription of BNIP3. These data, therefore, strongly argue that autophagy can, indeed, be controlled at the transcription level.
This review focuses on the links between autophagy and epigenetics in cancer to describe the following: (i) how ATG genes are regulated by epigenetics, including DNA methylation and post-translational histone modifications; (ii) how epidrugs are able to modulate autophagy in cancer and to alter cancer-related phenotypes (proliferation, migration, invasion, tumorigenesis, etc.) and; (iii) how epigenetic enzymes can also regulate autophagy at the protein level. One noteable observation was that researchers most often reported conclusions about regulation of the autophagy flux by epigenetic modifications or epidrugs, by only analyzing the levels of the LC3B-II form in treated cells. However, it is now widely accepted that an increase in the LC3-II form could be the consequence of an induction of the autophagy flux, as well as a block in the autophagosome-lysosome fusion and therefore vesicle degradation. We systematically reanalyzed all the published results describing the link between epidrugs and autophagy to determine whether autophagy flux was indeed regulated by epidrugs. To do so, we determined whether the conclusions of the authors were based on different protocols analyzing autophagy flux following a treatment with an epidrug (LC3B-II levels, number of autophagosomes in presence and absence of inhibitors of autophagy induction, and autophagosome-lysosome fusion, etc.) or whether the conclusions were only based on the analysis of the LC3B-II levels.
Therefore, to the best of our knowledge, this review summarizes, for the first time, the recent data describing a new approach to regulate autophagy during the development of cancers. These data clearly demonstrate that some cancer cells could profit from the use of a combination of epidrugs and autophagy inhibitors while, in other cancers, an increase of autophagy, which is frequently observed following the use of epidrugs, led to increased autophagy cell death.
2. Regulation of Autophagy Genes in Cancer Cells by DNA Methylation
Epigenetics is a transmissible but reversible process controlling gene expression. Among epigenetic modifications occurring in promoters, DNA methylation is a mark affecting DNA, whereas histone post-translational modifications modify the chromatin. DNA methylation and histone modifications both regulate gene transcription by modulating local chromatin structure and selective fixation of chromatin readers.
2.1. Basics of DNA Methylation
DNA methylation is the process leading to the addition of a methyl group onto the fifth carbon of a cytosine located in CpG motifs. About 80% of CpGs in the genome are methylated in mammals and this epigenetic mark is generally associated to gene repression and heterochromatin condensation. DNA methylation is catalyzed by a family of enzymes, called the DNA methyl transferases (DNMTs). On the one hand, DNMT1 mainly regulates the maintainance of DNA methylation on the newly synthetized DNA strand following DNA replication using the parental methylated strand as a matrix. DNMT3A and DNMT3B, on the other hand, are involved in de novo methylation on both stands of DNA, a process which is independent of the S-phase replication, and their roles during embryogenesis and inactivation of tumor suppressor genes (TSG) in cancers are well described. Another enzyme, DNMT3L, does not contain any catalytic domain but has been shown to be able to activate the latter enzymes. DNA methylation has been closely associated to tumorigenesis. For example, a global DNA hypomethylation is frequently observed in tumors and is correlated to grade. Local hypomethylation, as well as local hypermethylation, could also, respectively, lead to the expression of specific genes (e.g., oncogenes, antiapoptotic genes, etc.) or the specific inhibition of gene expressions (TSG, proapoptotic genes, etc.) (for a review, [4]).
2.2. Negative Regulation of Autophagy by DNA Methylation Favors Cancer Cell Aggressiveness
2.2.1. Molecular Mechanisms Inhibiting ATG Gene Expression via DNA Methylation
ChIPsequencing analyses showed in a prostate cancer cell model that the co-repressor DAXX (death domain associated protein) colocalized with DNMT1 near the TSS (transcriptional start site) of several ATG-related genes including ULK1 and DAPK3 (death-associated protein kinase 3) [5]. Since DAXX has already been shown to specifically recruit DNMTs on specific target promoters, these data strongly suggested that DAXX may actively mediate a methylation-dependent silencing of ATG genes [6]. Moreover, it has been shown that DNMT1 expression levels are strongly increased in childhood acute lymphatic leukemia (ALL) as compared with healthy individual samples. DNA digestion of ALL samples using restriction enzymes able to target modified CG sites showed a protection of digestion of ATG5 and LC3B promoters confirming CG methylation; it has been shown that the promoter methylation of these genes is correlated with decreased expression levels [7]. Interestingly, these publications also demonstrated that DNA methylation-dependent inhibition of ATG-related gene expression, in many different cancer models, led to the promotion of aggressiveness by reducing autophagy cell death.
2.2.2. Negative Regulation of ATG Genes by DNA Methylation in Cancer Cells
Several genes directly involved in the core of the autophagy process have been shown to be controlled by DNA methylation in cancers (these genes are listed in the Table 1). Indeed, the expression of BECN1 (Beclin 1) is frequently inactivated by a loss of heterozygosity (LOH) combined with a promoter hypermethylation in invasive breast cancers [8]. Similarly, ATG4D, ATG2B, ATG9A, and ATG9B promoter hypermethylation have been found in most of invasive ductal carcinomas (IDC) and this increased methylation was correlated to the grade of cancer, a decreased gene expression, and lymph node infiltration [9]. Hypermethylation of BNIP3 promoter (BCL2/adenovirus E1B 19 kDa protein-interacting protein 3) was also reported in colorectal carcinomas and a 5-aza-deoxycytidine (5-azadC) treatment restored its expression in colorectal cancer cells [10]. Similarly, combined 5-aza-dC and TSA (trichostatin A) treatments restored GL1 (GABARAPL1, GABA type A receptor-associated protein like 1) expression in breast cancer cell models [11]. In both gastric carcinoma and non-cancerous mucosae, methylation of the promoter of MAP1LC3v1 (microtubule-associated protein 1 light chain 3 alpha variant 1, also called LC3B) decreased its expression and was associated with Helicobacter pilori infection [12]. Since the inhibition of MAP1LC3v1 expression, in gastric cancer cells, favored proliferation, migration, and invasion of cancer cells, these results suggested that the methylation of MAP1LC3v1 might contribute to gastric carcinogenesis. The MAP1LC3Av1 gene, but not MAP1LC3B, was also silenced by methylation in oesophageal squamous cell carcinoma and overexpression of MAP1LC3Av1 in these cells decreased tumorigenesis in vivo [13].
Table 1.
Specific promoter methylation of ATG genes in cancers (ND, not determined).
| Epigenetic Regulation | Gene | Gene Expression | Cancer | Ref |
|---|---|---|---|---|
| DNA hypermethylation | ATG2B | Decreased | Invasive ductal carcinoma | [9] |
| ATG5 | Decreased | Childhood acute lymphatic leukemia | [7] | |
| ATG4D | ND | Invasive ductal carcinoma | [9] | |
| ATG9A | ND | Invasive ductal carcinoma | [9] | |
| ATG9B | Decreased | Invasive ductal carcinoma | [9] | |
| ATG16L1 | ND | Medulloblastoma | [14] | |
| BECN1 | Decreased | Invasive ductal carcinoma | [8] | |
| BNIP3 | Decreased | Colorectal cancer cell lines | [10] | |
| GL1 | Decreased | Breast cancer | [11] | |
| MAP1LC3 | Decreased | Lung cancer cell lines | [15] | |
| Childhood acute lymphatic leukemia | [7] | |||
| Gastric carcinoma | [12] | |||
| ULK2 | ND | Gastric carcinoma | [12] | |
| Decreased | Glioblastoma | [16] | ||
| DNA hypomethylation | ATG4A | Increased | Ovarian cancer cell lines | [17] |
2.2.3. Inhibition of DNA Methylation Rescue ATG Gene Expression and Reverse Cancer-Related Phenotypes
A similar methylation-dependent gene silencing was also reported in lung cancer cell lines, but not in lung cancers [15]. Indeed, EGFR-TKI-resistant (epidermal growth factor receptor-tyrosine kinase inhibitor) lung cancer cells presented a decreased methylation of the promoter of MAP1LC3v1 associated with increased LC3A protein expression [18]. Inhibition of LC3A expression using siRNA also decreased LC3B-II levels and cell proliferation, whereas a 5-azadC treatment restored LC3A expression in lung cancer PC9 cells and decreased their response to EGFR-TKI. ULK2 expression was also shown to be downregulated following promoter methylation in Glioma cell lines and was restored following a 5-azadC treatment [16]. Moreover, overexpression of ULK2 strongly induced autophagy-mediated cell death in a reactive oxygen species (ROS)-dependent manner. Indeed, treatment of ULK2-overexpressing cells with a ROS scavenger, N-acetyl cysteine (NAC), or an inhibitor of the early steps of autophagy, 3-MA (3-methyladenine), blocked ULK2-induced cell death. Expression of LAPTM5 gene (lysosomal-associated protein multispanning transmembrane 5) coding a protein associated to lysosomes was downregulated in all (10 out of 10) neuroblastoma tested. LAPTM5 expression was inversely correlated to promoter methylation and this hypermethylation could be reverted by a 5-azadC treatment [19]. Indeed, LAPTM5 overexpression provoked a caspase-independent cell death associated with an accumulation of autophagic vesicles. However, since neither wortmannin, an autophagy inhibitor, nor siATG5 affected LAPTM5-induced cell death, the authors proposed that classical autophagy cell death was not involved. Indeed, LAPTM5 overexpression led to lysosomal destabilization and blocked the autophagy flux, and thus led to cell death [19]. Surprisingly, a hypomethylation of the ATG4 promoter was frequently associated with the increased expression of the gene and poor prognosis in ovarian carcinoma patients [17].
2.2.4. Indirect Regulation of Autophagy by DNA Methylation in Cancer Cells
The tumor suppressor gene (TSG) PCDH17 (Protocadherin 17), which is an activator of autophagy, has been shown to be frequently silenced by promoter hypermethylation in gastric and colorectal cancers, but not in normal tissues [20]. Similarly, in hepatocellular carcinoma samples, methylation of the promoter of BCL2L10 (Bcl-2-like protein 10) was associated with a decreased expression of BCL2L10 [21]. Overexpression of this protein in hepatoma cells restored autophagy in an AMPK-manner and inhibited cell proliferation and tumor growth in mice models. In glioblastoma cells, the TSG ANKDD1A (ankyrin repeat and death domain containing 1A) was also frequently silenced by hypermethylation [22]. In normal cells, ANKDD1A interacts with, and activates the expression of FIH1 (factor inhibiting HIF1) which leads to the downregulation of HIF-1α, and thus to decreased autophagy and induction of cell death. Indeed, activation of the HIF-1-dependent autophagy pathway in GBMs has been previously demonstrated to be a survival pathway.
2.2.5. Molecular Mechanism Controlling Cancer Cell Aggressiveness in a DNA Methylation-Mediated ATG Repression Manner
The treatment of ovarian carcinoma cells with 5-azadC has been shown to induce autophagy and promote cell death [23]. These effects are highly potentiated when 5-azadC is used together with SAHA (suberanilohydroxamic acid), an HDACi. The increase in autophagy in these cells has been demonstrated to be dependent of the induction of expression of the TSG ARH1 (age-related hearing impairment 1). A treatment of colorectal cancer cells (HT-29) with Se-allylselenocysteine (ASC) has been shown to induce a demethylation of the PCDH17 promoter associated with an increase in PCDH17 expression and an activation of autophagy [24]. This effect was described to be linked to the inhibitory effect of ASC on global DNA methylation and DNMT1 expression. Moreover, ASC has been described to significantly decrease HT-29 tumor xenograft growth in mice. It was also recently demonstrated that the activation of CDK4 (cyclin dependent kinase 4), which is frequently observed in cancer cells and linked to the inhibition of senescence, directly phosphorylates DNMT1 and blocks its degradation by autophagy [25]. On the contrary, inhibition of CDKs with palbociclib in prostate cancer cells led to the decrease in DNMT1 levels and overall methylation and contributed to the reduction of the resistance to senescence. Therefore, it has been proposed that the use of CDK inhibitors could be useful in the future to both restore autophagy, inhibit global DNA methylation, and counteract the activation of E2F target genes instead of combining multiple molecules and increasing off-targets [26].
3. Histone Deacetylases (HDACs) are Key Regulators of Autophagy
3.1. Basics of Histones Acetylation and Deacetylation
Lysine acetylation of histones 3 and 4 are well described epigenetics marks linked to the activation of gene transcription. These modifications are added by histone acetyl transferases (HAT) and removed by histone deacetylases (HDACs). However, it has to be kept in mind that acetylation of nonhistone proteins is also controlled by the same enzymes. To our knowledge, 18 HDACs have been reported in the mammalian genome and these proteins have been divided into the following four classes: class I includes HDAC1, HDAC2, HDAC3, and HDAC8 whichis generally localized in the nucleus; class II is composed of HDACs which shuttle between the cytoplasm and the nucleus and are subdivided into class IIa (HDAC4, HDAC5, HDAC7, and HDAC9) and class IIb (HDAC6 and HDAC10); class III comprises SIRT1 (Sirtuin-1), SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7; and class IV includes HDAC11. Classes I, II, and IV regroup zinc-dependent HDACs, whereas class III contains nicotinamide adenine dinucleotide (NAD+)-dependent HDACs. In cancer cells, acetylation and deacetylation of many promoters has been involved in the specific control of gene expression. Indeed, HDACs have been implicated in different key processes of cancer, including proliferation, inhibition of cell differentiation, cell death, angiogenesis, immune evasion, and autophagy (for a review, [27]). Moreover, HDACis, by modulating these phenotypes, are promising drugs to fight against cancer cells.
3.2. HDAC Activities Control Autophagy
The effects of the modulation of HDAC expression or HDAC activity on autophagy has been extensively investigated in cancer cells. Altogether these data, compiled in the Table 2 and detailed below, suggest that HDACs play a key role in the control of autophagy and cancer-related phenotypes associated to autophagy. Moreover, a decrease in acetylation of the lysine 16 of histone 4 (H4K16ac) has been fully correlated to autophagy induction in physiologic conditions and mediated by a loss of HAT hMOF [28]. Indeed, repression of hMOF was dependent of the autophagy process, since blockade of autophagy using BafA1 or 3-MA restores hMOF content. Activation of AMPK, following glucose deprivation, resulted in phosphorylation of acetyl coA synthetase ACSS2 at S659 and modification of its three-dimensional (3D) structure leading to exposition of a NLS domain [29]. P-ACSS2 could interact with importin α5 that promoted importin α/importin β/ACSS2 complex formation, and thus favored ACSS2 nuclear translocation and its further association with TFEB. The ACSS2/TF complex can incorporate acetate from histone acetylation turnover to produce locally acetylcoA and used it to induce H3 acetylation in promoters of ATG-related genes, thus, promoting both autophagy (e.g., activation of lysosome genes CTSA (coding cathepsin A), GBA (β-glucuronidase), GUSB (β-glucosidase), and LAMP1 (lysosomal membrane protein 1) and cell survival. Indeed, invalidation of ACSS2 expression inhibited cancer cell growth and tumorigenesis in rodent models [30].
Table 2.
Described effects of epidrugs on autophagy in cancer cells. Classification from left to right: Epigenetic target, epidrug; cancer cells used in the studies; cancer origin; and effect on autophagy.
| Target | Epigenetic Drug | Cells | Cancer Origin | Autophagy | Ref |
|---|---|---|---|---|---|
| BETi | JQ1 | KP-4 | Pancreas carcinoma | Increase ATG gene expression | [31] |
| DNMT | 5-aza-dC | Hey | Ovarian carcinoma | Increase of AVOs | [23] |
| K-562 | Chronic myeloid leukemia | Increase of LC3B-II | [32] | ||
| EZH2 | DZNep | RKO, HCT116 | Colorectal carcinoma | Increase of LC3B-II | [33] |
| GSK126 | MG803 | Gastric carcinoma | Increase of LC3B-II and decrease of phospo AKT/mTOR/ULK1 | [34] | |
| GSK343 | U2OS | Bone carcinoma | Increase of LCB-II, decrease of P62/SQSTM1 | [35] | |
| HCT115, DLD-1 | Colorectal carcinoma | Increase of LC3B flux and autophagic vesicles | [36] | ||
| MDA-MB-231 | Triple negative breast cancer | Increase of LC3B and SQSTM1/P62 fluxes | [37] | ||
| 1o | K562 | Myelogenous leukemia | Increase of LCB-II | [38] | |
| SK-N-BE | Neuroblastoma | Increase of LCB-II | [38] | ||
| UNC1999 | HT-29, HC-T15 | Colon cancer | Increase LC3B-II flux | [39] | |
| Lovo HCT115, DLD-1 | Colorectal carcinoma | Increase LC3B-II flux and autophagic vesicles | [36] | ||
| G9a | BIX01294 | U2OS | Bone carcinoma | Increase of LC3B-II and vesicles | [35] |
| HeLa | Cervix carcinoma | Increase of LC3B-II and vesicles | |||
| MCF-7 | Breast (LumA) | Increase of BECLIN-1 | [40] | ||
| TCA8113 | Tongue squamous cell carcinoma | Increase of LC3B-II | [41] | ||
| BE(2)-C, SHEP1 | Neuroblastoma | Increase of vesicles and LC3B-II | [42] | ||
| HCT116 | Colorectal carcinoma | Increase of vesicles and LC3B-II, activation of ATG gene expression | [43] | ||
| Kaempferol | AGS | Gastric carcinoma | Increase of LCB-II, decrease of P62/SQSTM1, autophagic cell death | [44] | |
| HDAC | Apicidin | YD-8,YD-10B, AT84 | Oral squamous carcinoma | Increase of LC3B-II, ATG5 and AVOs | [45,46] |
| YD-15 | Mucoepidermoid carcinoma | Increase of LC3B-II and AVOs, decreased P62 | [47] | ||
| Butyrate | HeLa | Cervix carcinoma | Increase of vesicles and autophagic cell death | [48] | |
| ITF2357 (givinostat) | U87, U251 | Glioblastoma | Increase of LCB-II, ATG5, ATG7, BECLIN-1, autophagic cell death | [49] | |
| MGCD0103 | Primary B-cell chronic lymphocytic | B-cell chronic lymphocytic leukemia | decrease of LCB-II, ATG5, ATG12, P62/SQSTM1, BECLIN-1 and of autophagic flux | [50] | |
| MHY218 | MCF-7 | Breast (LumA) | Increase of LCB-II and BECLIN-1 | [51] | |
| Panobinostat (LBH589) | L428, L540 | Hodgkin lymphoma | Increase of LCB-II and vesicles | [52] | |
| Huh7 | Hepatocarcinoma | Increase of LCB-II and decrease of P62/SQSTM1 | [53] | ||
| MDA-MB-231, SUM159PT | Triple negative breast cancer | Increase of LCB-II, BECLIN-1 and decrease P62/SQSTM1 | [54] | ||
| OSU-HDAC42 | HCCs | Hepatocarcinoma | Increase of LCB-II and vesicles | [55] | |
| SAHA (Vorinostat) | HeLa | Cervix carcinoma | Increase of vesicles | [48] | |
| RCS, OUMS-27 | Chondrosarcoma | Increase of LCB-II and vesicles | [56] | ||
| HCCs | Hepatocarcinoma | Increase of LCB-II and vesicles | [55] | ||
| T98G, U251MG, C6 | Glioblastoma | Increase of LCB-II, vesicles and AVOs | [57,58] | ||
| MCF-7, MCF-7 Tamox-R | Breast (LumA) | Increase of LCB-II, BECLIN-1, vesicles, AVOs, autophagic cell death and decrease of P62/SQSTM1 | [59,60] | ||
| MDA-MB-231 | Triple negative breast cancer | Increase of LCB-II, BECLIN-1 and AVOs and decrease of P62/SQSTM1 | [60] | ||
| HUT78 | T-cell lymphoma | Increase of LCB-II | [61] | ||
| MYLA | Cutaneous T-cell lymphomas | Increase of LCB-II | [61] | ||
| A2058, A375 | melanoma | Increase of LCB-II | [61] | ||
| HCT116, HCT15 | Colorectal carcinoma | Increase of LCB-II, ATG5 | [61] | ||
| µ-myc | B-cell lymphoma | Increase of LCB-II | [62] | ||
| 4T1 | Breast cancer | Increase of vesicles | [63] | ||
| Sulforaphane | MDA-MB-231, BT549, MDA-MB-468 | Triple negative breast cancer | Increase of LCB-II, BECLIN-1 and vesicles and decrease of P62/SQSTM1 | [64] | |
| ZW2-1 | HL-60 | Leukemia | Increase of LCB-II, vesicles | [65] | |
| HDAC (class I/II) | FK228 | KP-MRT-NS | Malignant rhabdoid tumors | Increase of LC3B-II and vesicles, autophagic cell death | [66] |
| Valproate | A2058, A375 | Melanoma | Increase of LCB-II | [61] | |
| Namalwa, Raji, Daudi, Ramos | Burkitt leukemia/lymphoma | Increase of LCB-II, BECLIN-1, vesicles and decrease of P62/SQSTM1, P-MTOR | [67] | ||
| CMK | Acute megakaryocytic leukemia | Decrease of ATG7 mRNA and increase of GFP-LC3B vesicles | [68] | ||
| Jurkat, H9 | T-lymphoma | Increase of LCB-II, vesicles and decrease of P-MTOR | [69] | ||
| SU-DHL-4 | B-lymphoma | Increase of LCB-II, vesicles and decrease of P-MTOR | [69] | ||
| HepG2 | Hepatocarcinoma | Increase of LCB-II and AVOs | [70] | ||
| HeLa | Cervix carcinoma | Increase of LC3B vesicles | [28] | ||
| JMJD2 | ML324 | U2OS | Bone carcinoma | Increase of LCB-II | [35] |
| KDM5B | PBIT | U2OS | Bone carcinoma | Increase of LCB-II and P62/SQSTM1 | [35] |
| KDM6B/JMJD3 | GSKJ4 | U2OS | Bone carcinoma | Increase of LCB-II, decrease of P62/SQSTM1 | [35] |
| LSD1 | GSK-LSD1 | U2OS | Bone carcinoma | Increase of LCB-II, decrease of P62/SQSTM1 | [35] |
| S2101 | SKOV3 | Ovarian carcinoma | Increase of LCB-II, GFP-LC3B vesicles and decrease P62/SQSTM1 | [71] | |
| JL1037 | THP-1, Kasumi-1 | Acute myeloid leukemia | Increase of LCB-II and vesicles | [72] | |
| NCL1 | LnCAP | Prostate carcinoma | Increase of LCB-II flux, vesicles | [73] | |
| SP2509 | SHSY5Y | Neuroblastoma | Increase of LCB-II | [74] | |
| ARK2, TOV112D | Ovarian carcinoma | Increase of LCB-II, ATG7 and P62/SQSTM1 | [75] | ||
| TCP | SHSY5Y | Neuroblastoma | Increase of LCB-II and GFP-LC3B vesicles | [74] | |
| HO8910 | Ovarian carcinoma | Increase of LCB-II | [76] | ||
| SIRT1 | Sirtinol | MCF-7 | Breast (LumA) | Increase of LCB-II and AVOs | [77] |
| J11-C1 | SKOV3 | Ovarian carcinoma | Increase of LCB-II and BECLIN-1 | [78] | |
| 15dPGJ2 | SKOV3 | Ovarian carcinoma | Increase of LCB-II | [78] | |
| J19 | SKOV3 | Ovarian carcinoma | Increase of LCB-II, ATG3 and BECLIN-1 | [78] | |
| MHY2256 | Ishikawa | Endometric carcinoma | Increase of LCB-II, ATG5/7, BECLIN-1, AVOs | [79] | |
| SKVO3 | Breast (LumA) | Increase of LCB-II and AVOs | [80] | ||
| MCF-7 | Ovarian carcinoma | Increase of LCB-II and AVOs | [80] | ||
| NCO-90/NCO141 | HL60, MT2, SIT1, Jurkat | Leukemia/T-lymphoma | Increase of LCB-II | [81] | |
| SIRT6 | UBCS039 | H1299 | Non-small cell lung carcinoma | Increase of LCB-II, GFP-LC3B vesicles and autophagic flux | [82] |
| HeLa | Cervix carcinoma | Increase of LCB-II, GFP-LC3B vesicles and autophagic flux | [82] |
Interestingly, acetylation of nonhistone proteins can also control the degradation of specific HDAC targets by autophagy leading to cancer aggressiveness. For example, PCAF (p300/CBP-associated factor) has been shown to favor the acetylation of δ-CATENIN and promote its degradation by autophagy and, consequently, reduce cell growth and mobility [83]. Indeed, overexpression of HDAC1, HDAC4, or HDAC8 markedly increases δ-CATENIN stabilization, to the same levels as the ones observed with autophagy inhibitors (CQ or 3-MA), whereas the inhibition of HDACs (classes I and II) with TSA decreases its stability. In the same way, FOXO1 and 3 transcription factors are effectively described to control gene expression of proteins implicated in autophagosome formation, direct deacetylation of FOXO 1 and 3 by sirtuins induces nuclear localization and increases they ability to bind to DNA [84,85].
3.3. HDACi (Classes I and II) Promote Autophagy Cell Death in Cancer Cells
The inhibition of HDACs, using HDACi, has been associated with an increase of autophagy signaling which could lead to autophagy-linked cell death in many different cancer models (Figure 2). Indeed, SAHA or butyrate promotes both apoptosis and autophagy-induced cell death in HeLa or chondrosarcoma cells (RCS, OUMS-27) [48,56]. Similarly, a molecular mechanism explaining the induction of autophagy by SAHA in breast cancer cells has recently been proposed [60]. Indeed, SAHA has been described to repress the transcription of the autophagy repressor gene SURVIVIN, as well as promote the acetylation of the protein leading to its nuclear translocation. Since nuclear SURVIVIN is less stable than the cytosolic protein and more rapidly degraded, these data might explain how SAHA could favor autophagy. Moreover, inhibition of SURVIVIN expression using siRNA in breast cancer cells (MCF-7 and MDA-MB-231) also promoted autophagy, whereas overexpression of SURVIVIN decreased LC3B puncta [60]; siHDAC2, siHDAC3, and siHDAC6 also decreased SURVIVIN expression and promoted LC3B-II conversion in these cells, whereas siHDAC1 led to opposite results [60]. Interestingly, SAHA is known to be more specific towards HDAC3 and HDAC6 than towards HDAC1, and HDAC6 has already been described to be involved in the acetylation of SURVIVIN on K129. Similarly, panobinostat inhibited cell proliferation and promoted both apoptosis and autophagy in Huh7 hepatocellular carcinoma cells [53]. Moreover, depletion of HDAC1 in hepatocellular cancer cells provoked a decrease in cell proliferation and an activation of autophagy-induced cell death [86]. Another HDACi, ZW2-1, also promoted both mitochondria-mediated apoptosis and the number of autophagy vesicles or the levels of LC3B-II in leukemia HL-60 cells [65].
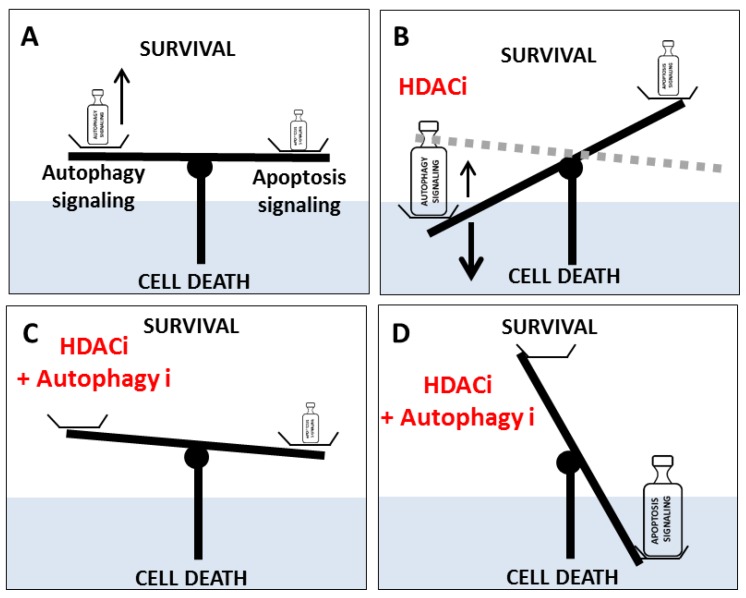
Figure 2.
Model of the balance between autophagy and apoptosis governing cell death. (A) In cancer cells, the combination of an autophagy-mediated pro-survival signal and weak apoptosis led to cancer cell survival. (B) An HDACi can promote a slight increase in apoptosis signaling but also a strong increase in autophagy signaling leading to a balance in favor of autophagy-linked cell death (solid line) or survival (dotted line) depending of both cancer cell model and autophagy induction level. (C) The combination of an HDACi and an autophagy inhibitor (autophagy i) in cells resistant to apoptosis, or in the absence of an external signal of apoptosis, led to a balance in favor of survival via the inhibition of autophagy-linked cell death. (D) The combination of an HDACi and an autophagy inhibitor (autophagy i) led to a balance in favor of apoptosis.
Interestingly, VPA (valproic acid) and SAHA induced the expression of UVRAG in HCT116 colorectal cells and then limited apoptosis in response to 5-FU [87]. Inhibition of autophagy (with CQ or in an ATG7 knockdown) also enhanced anticancer properties of SAHA, decreased cell proliferation and induction of apoptosis in chronic myeloid leukemia cells, or of vorinostat in colorectal cells in a ROS-dependent manner [88,89]. Indeed, ROS production caused by these compounds led to an accumulation of ubiquitinylated proteins and, then, to increased cell death. Indeed, in U2OS cells, ubiquitination of proteins following SAHA treatment was dependent of the protein HR23B (RAD23 (S. Cerevisiae) homolog B) [61] and it has been shown that a decrease of HR23B levels, decreased ubiquitination-mediated apoptosis. All these data, therefore, support that Class I and II HDACi alone can promote autophagy and led to autophagy-linked cell death in a ROS-dependent manner in many different cancer models.
3.4. Inhibition of Autophagy Induced by HDACi Blocks Cell Death
SAHA or OSU-HDAC42 also induced autophagy and autophagy-mediated cell death in hepatocellular carcinoma cells by repressing the AKT/mTOR signaling pathway [5]. However, HDACi-induced cell death could be limited by the addition of the autophagy inhibitor 3-MA or by the inhibition of ATG5 expression [55]. In Burkitt leukemia and lymphocyte cell lines, VPA also promoted autophagy, and its combination with a mTOR inhibitor, temsirolimus, further increased autophagy, inhibited cell growth, and synergistically promoted autophagy-mediated cell death [67]. Moreover, inhibition of autophagy using 3-MA, BafA1, or siATG5 decreased growth inhibition mediated by the combined treatment, suggesting that autophagy could act as an antiproliferative pathway in this model. In highly metastatic mice breast cancer cells (4T1), SAHA also increased autophagy (accumulation of LC3B-II and induction of BECLIN1 expression) and potentiated radiation-induced cell death [63]. Indeed, blocking autophagy, using 3-MA, decreased toxicity of the combined treatment. In lung cancer cell lines (PC-9G and H1975), presenting a resistance to EGFR tyrosine kinase inhibitors due to the mutation T790M, SAHA restored the sensitivity to these inhibitors by promoting both apoptosis and autophagy-linked cell death [90]. Inhibition of autophagy by overexpression of spautin-1, which triggers the ubiquitinylation of BECLIN-1, or with siLC3B or siBECN1, decreased both caspase-dependent and independent apoptosis induced by the combined EGFR tyrosine kinase inhibitor/SAHA treatment in these cells. VPA also potentiated the formation of LC3B-GFP vesicles induced by the multi-kinase inhibitor Pazopanib in sarcoma cell lines. Inhibition of autophagy using siBECN1 or siATG5 abrogated VPA/Pazopanib treatment-induced cell death [50]. Romdepsin, another HDACi, also potentiated the response to Bortezomib in gastric carcinoma cells by inhibiting cell proliferation and increasing both apoptosis and autophagy-mediated cell death [91]. Indeed, addition of a ROS scavenger or inhibition of autophagy, using 3-MA, strongly decreased autophagy-induced cell death in these cells (AGS-BDneo and SNU-719) suggesting that the induction of ROS-dependent autophagy was required for the efficiency of the combined treatment [91]. Similarly, AR42, a classI/II HDACi, potentiated cell toxicity of the multi-kinase inhibitor Pazopanib in drug-resistant melanoma cells [92]. Toxicity was mediated by increased death receptor signaling, ER stress signaling and, mainly, autophagy induction (accumulation of BECLIN-1 and positive phosphorylation, S318-P-ATG13, S317-P-ULK1, T172-P-AMK in detriment of negative phosphorylation, S757-P-ULK1, S2448, and S2481-P-mTOR). Moreover, inhibition of autophagy via siBECN1 or siATG5 decreased AR42 toxicity. Treatment of T/B-lymphoma cell lines with a combined treatment VPA/doxorubicin increased autophagy (increased LC3B-II and BECLIN-1 levels and autophagy vesicle number) via the activation of AMPK and the inhibition of mTOR, leading to cell toxicity [69]. Inhibition of autophagy in Jurkat cells using drugs (3-MA or BafA1), siRNA (BECN1 or ATG5) but not with the caspase inhibitor ZVAD-FMK strongly reduced anti-cell growth effects. Surprisingly, the inhibition of HDAC1 or HDAC3 expression, using RNAi, had no effect on the induction of autophagy by VPA/doxorubicin, suggesting that this process was HDAC-independent. Indeed, VPA induced a drop in IP3 levels, and thus a blockade of calcium in mitochondria leading to autophagy, independently of HDAC activity [69]. Similar observations reported that VPA/doxorubicin cotreatment synergistically inhibited cell viability in HepG2 cells, by promoting both apoptosis and autophagy-mediated cell death in a ROS production-dependent manner, cell death which could be reversed by addition of NAC or 3-MA [70]. Pemetrexed/sildenafil (P/S) cotreatment also increased autophagy in non-small cell lung carcinoma (NSCLC) cells (A549) via an induction of CerS6 (ceramide synthase 6) expression and the disruption of HDAC6 content [93]. The inhibition of autophagy by siBECN1 or siATG5 decreased P/S-induced toxicity, whereas a cotreatment of P/S with AR42 or VPA increased cell death. Similarly, the combination of VPA with neratinib increased NSCLC cell toxicity by inactivating mTORC1 and mTORC2, and thus inducing both autophagy and cell death [94]. Moreover, inhibition of BECN1 or ATG5 expression, or forced expression of an active form of mTOR severely decreased cell death. Cotreatment of uveal melanoma cells with neratinin/MS275 (entinostat) also strongly reduced cell viability by inducing apoptosis and autophagy-linked cell death [95]. Indeed, neratinin/MS275 activated AMPK/ULK1 and ATG13 phosphorylation in a ROS-dependent manner and also severely reduced HDAC6 content leading to cell death. Moreover, overexpression of mTOR significantly reverted cell toxicity mediated by the neratinin/MS275 cotreatment [95]. Indeed, these data strongly suggest that in many cancers, inhibition of autophagy decreased cell death and toxicity mediated by anticancer molecules. As expected, all these data clearly showed, that the inhibition of autophagy blocked autophagy-linked cell death and may favor cancer cell models (Figure 2).
3.5. HDACi Treatments Modify the Balance Between Autophagy and Apoptosis
3.5.1. Effects of SAHA and VPA
Although HDACi promote autophagy, which can induce autophagy-mediated cell death (see above), these molecules also act on apoptosis signaling. Indeed, a balance between autophagy and apoptosis has frequently been observed. Since autophagy signaling, following HDACi treatment, can compete with chemical-induced apoptosis for cell survival/death, much data have confirmed that the inhibition of autophagy can promote cell death mediated apoptosis (Figure 2). Indeed, inhibition of autophagy using siBECN1 in hepatoma cell lines (HepG2, Hep3B, and PLC/PRF/5) enhanced apoptosis induced by the cotreatment SAHA-sorafenib (multi-kinase inhibitor) [96]. Similarly, blockade of panobinostat-induced autophagy using CQ in triple negative breast cancer cells lines (MDA-MB231 and SUM159PT) also led to the accumulation of ubiquitinylated proteins such as SQSTM1/P62-Ub and promoted both cell death in vitro and decrease of tumor growth in xenograph models [54]. In T47D ER (estrogen receptor) positive breast cancer cells, VPA favored apoptosis instead of growth arrest when cells were treated with tamoxifen [97]. However, tamoxifen resistant T47D cells presented a higher autophagy activity, and the inhibition of autophagy in these cells using CQ or BECN1 silencing restored cell death. In the breast cancer MCF-7 cell line, inhibition of autophagy using 3-MA also potentiated the antiproliferative effect of HDACi, such as CTS203, and promoted apoptosis via the CASPASE 8-mediated cleavage of BECLIN-1 [98]. However, in MCF-7-tamoxifen resistant cells, SAHA alone was sufficient to promote autophagy-dependent cell death [59]. Similar results were obtained in glioblastoma, since the transfection of siATG7 in T98G cells blocked the induction of SAHA-induced autophagy which was associated to increased LC3 expression and impaired mTOR signaling, and thus favored apoptosis [57]. Combined SAHA/CQ treatments also promoted apoptosis in glioblastoma cell models [99]. Surprisingly, inhibition of the early steps of autophagy using 3-MA, decreased SAHA/CQ-induced cell death suggesting that the accumulation of autophagosomes could be required to induce cell toxicity. These results, therefore, suggest that the benefits of autophagy inhibition against cancer cells are not due to a negative regulation of a pro-survival pathway but rather are dependent on the production of ROS and mitochondria accumulation leading to cell death [99]. Indeed, other data showed that the combination of SAHA with quinacrine, an anti-malaria and autophagy inhibitor, also promoted apoptosis in T-cell leukemia Jurkat cell line by decreasing mitochondrial membrane potential and inhibiting mitophagy [100]. Moreover, a quinacrine/SAHA cotreatment led to ubiquitinylated mitochondrial aggresomes and cell death, effects which could be reverted using the antioxidant NAC.
3.5.2. Others HDACi
Another HDACi, apicidin, also promoted both apoptosis and autophagy in oral squamous cell carcinoma cells and in mucoepidermoid carcinoma YD-15 cells [45,46,47]. Inhibition of autophagy, using CQ, in these cells further increased cell death linked to the inhibition of the phosphorylation of both AKT and mTOR, the accumulation of LC3B-II, ATG7, and a decrease of SQTM1/P62 levels [45,46,47]. Since apicidin seemed to specifically inhibit HDAC8, a protein frequently overexpressed in oral squamous cell carcinoma tumors, HDAC8 signaling appeared important for the regulation of cell proliferation in this model. Inhibition of HDAC classes I-II in rhabdoid tumor cells, using FK228, also induced autophagy and cell death but the combined inhibition of HDAC and autophagy, with the additional use of CQ, further increased cell death suggesting that autophagy could partially protect these cells from death [66]. Similarly, CQ also increased VPA-induced toxicity in AML cells [101]. A specific inhibition of HDAC8 expression by siRNA in oral squamous cell carcinoma cells (YD-10B and FaDu) also promoted both apoptosis and an increase in BECLIN-1, ATG5, ATG12, LC3B-II, and acidic vesicle number, whereas the inhibition of autophagy, using CQ, dramatically increased siHDAC8-induced cell toxicity [102]. The inhibitor of HDAC, MGCD0103, promoted apoptosis in primary chronic lymphocytic leukemia (CLL) in detriment of autophagy [50]. Indeed, MGCD0103 activated the PI3K/AKT/mTOR signaling pathway, decreased ATG gene expression (such as BECN1, UVRAG, ATG7, ATG12, or GABARAP), and thus decreased autophagy flux in primary chronic lymphocytic leukemia, but not in PBMCs (peripheral blood mononuclear cells). However, inhibition of autophagy, using 3-MA or CQ, in primary chronic lymphocytic leukemia inhibited cell growth, whereas combined treatment with MGCD0103 (or VPA) and autophagy inhibitors potentiated cell death [50]. Surprisingly, the blockade of vorinostat-induced autophagy using shRNA ATG5 or ATG7, in Eµ-myc lymphoma cells, had no effect on cell death [62]. These data suggested that, in this model, autophagy induction and cell death mediated by vorinostat were two independent processes.
In 2014, a phase I study tested the combination of SAHA and hydroxychloroquine (HCQ) for the treatment of solid tumors [103]. This study established a maximum daily tolerated dose of 600 mg HCQ and 400 mg SAHA. Among the included patients, one with renal carcinoma presented a stable partial response to treatment. On the basis of the in vitro results, a combination of SAHA with CQ remains promising but efforts are needed to specifically identify the population that could benefit from this treatment. Some additional clinical trials in solid tumors, testing the combination of HDACi and derivative CQ, are currently active or in recruitment (Table 3) (clinicaltrials.gov).
Table 3.
Clinical trials testing autophagy inhibitor and HDACi.
| Trial Reference | Study | Cancer | Drugs | Status |
|---|---|---|---|---|
| NCT01023737 | Hydroxychloroquine + vorinostat in advanced solid tumors | Malignant solid tumor | Hydroxychloroquine Vorinostat |
Active, not recruiting |
| NCT01266057 | Sirolimus or vorinostat and hydroxychloroquine in advanced cancer | Advanced cancers | Hydroxychloroquine Sirolimus Vorinostat |
Active, not recruiting |
| NCT03243461 | International cooperative phase III trial of the HIT-HGG study group (HIT-HGG-2013) | Glioblastoma WHO Grade IV Diffuse Midline Glioma Histone 3 K27M WHO Grade IV Anaplastic Astrocytoma WHO Grade III |
Temozolomide + Valproic Acid Temozolomide + Chloroquine |
Recruiting |
| NCT02316340 | Vorinostat Plus Hydroxychloroquine versus regorafenib in colorectal cancer | Colorectal cancer | Vorinostat Hydroxychloroquine Regorafenib |
Active, not recruiting |
3.5.3. Effect of HDACi/Autophagy and Chemotherapy Combination on Cancer Cells
The inhibition of autophagy, using CQ, severely potentiated cell toxicity mediated by TMZ, SAHA alone, or the combined TMZ/SAHA treatment in glioma cells (C6, U251MG) [58]. Similarly, CQ also increased the effects of SAHA in the glioma GL261 rodent model, suggesting that autophagy induced by SAHA was a protective response against cell death in this type of cancer [58].
In Down syndrome-associated myeloid leukemia cells, VPA treatment was associated with a downregulation of 409 autophagy-related genes, associated with an inhibition of autophagy flux and an accumulation of ROS and mitochondria [68]. In MCF-7 cells with acquired resistance to tamoxifen, the HDACi, MHY218, promoted cell arrest and autophagy cell death and suppressed tumor growth in vivo.
Moreover, cotreatment of TNBC cells (MDA-MB-231 and MDA-MB-468) with LBH549, an HDACi, and Mevastatin, a HMGCR (3-hydroxy-3-methylglutaryl-CoA reductase) inhibitor, increased apoptosis and reduced tumor growth in nude mice models as compared with effects obtained with the molecules used alone [104]. Indeed, this cotreatment induced autophagy by activating the T172-P-AMPK phosphorylation and inhibiting mTOR and P70S6K but at the same time, also inhibited autophagy flux by preventing the formation of the Vsp34/BECLIN-1 complex and decreasing the prenylation of Rab7 (an active form of the small GTPase involved in autophagosome-lysosome fusion) [104]. SAHA and Olaparib, a PARP (poly (ADP-ribose) polymerase 1) inhibitor, cotreatment decreased cell viability in triple negative breast cancer cells (MDA-MB-157, MDA-MB-231, and HCC1143) but not in MDA-MB-468 and HCC70 cells [105]. Indeed, these differences could be explained by the different levels of PTEN (phosphatase and TENsin homolog) in these cells. Moreover, inhibition of PTEN levels using siRNA in MDA-MB-231 cells strongly decreased BECLIN-1 and LC3B contents and autophagy vesicle number.
3.6. Specific Inhibition of Class II HDAC also Promote Autophagy Cell Death
Since panHDACi seem to promote autophagy-mediated cell death, some studies focused on the role of specific HDACs in this process. Indeed, HDAC4 has been described to deacetylate STAT1 (signal transducer and activator of transcription 1), and thus suppress autophagy in diabetes models [106]. Moreover, in multiple myeloma cells, miR-29b specifically targets HDAC4 mRNA and inhibits HDAC4 expression resulting in the inhibition of cell migration and cell viability and the induction of autophagy [107]. Indeed, treatment of these cells with the pan-HDAC inhibitor, SAHA, promoted miR29b expression and blocked pro-cancer HDAC4-associated phenotypes. Interestingly, a combined treatment of a miR29b mimics and SAHA synergistically reduced cell aggressiveness in vivo [107]. Inhibition of HDAC5 in HeLa cells using siRNA modulates ROS-related gene expression and provokes an important ROS production leading to apoptotic cell death and a mechanism of elimination of damaged mitochondria by mitophagy. HDAC5 inhibition induces also a metabolic reprogrammation towards glucose and glutamine. Concomitant inhibition of HDAC5 and glutaminase synthase using LMK235 and BTPE, respectively, reduced significantly tumor growth in vivo [108,109].
3.7. HDAC6 and HDAC10 are Pro-Autophagy Proteins Which Act at the Protein Level
In contrast, HDAC6 is a cytosolic protein favorable to autophagy. HDAC6-/Y mice present lower autophagy than wild type mice [110]. Moreover, HDAC6 can directly interact with HR23B and silencing of HDAC6 in NSCLC A549 cells, using RNAi, promotes both HR23B stabilization and global protein ubiquitination leading to reduced autophagy but increased apoptosis [61]. HDAC6 is also frequently overexpressed in GBMs and high expression of this protein is associated with poor prognosis. Indeed, HDAC6 KO sensitizes the U87 GBM cell line to radiotherapy-mediated autophagic cell death. Indeed, inhibition of autophagy using siBECN1 or 3-MA restores the formation of colonies in these cells [111]. Nutriment deprivation frequently occurs in solids tumors and its mimics in U87 cells leads to the accumulation of TDP-43 (TAR DNA binding protein), pro-cancer phenotypes and activation of a HDAC6 dependent autophagy at the expense of apoptosis [112]. Indeed, inhibition of HDACs using SAHA or inhibition of autophagic flux using 3-MA or BafA1 in TDP-43 overexpressing cells restores apoptosis. In melanoma cells, AR42 seems to specifically promote HDAC6 degradation by autophagy [113]. However, sulforaphane, a natural HDACi decreases HDAC6 expression at the mRNA and induces TNBC cell growth arrest and autophagy [64]. Indeed, a decrease of HDAC6 content promotes acetylation and activation of PTEN. PTEN silencing using specific siRNA reverses autophagy markers expression, suggesting that sulforaphane-induced autophagy activation is mediated by PTEN [64]. Although little is known concerning the role of HDAC7 on autophagy, its inhibition in mucoepidermoid carcinoma cells, using siRNA also blocks cell proliferation and promotes autophagy (accumulation of LC3B-II and acidic vesicles and decrease of SQSTM1/P62 content) [114].
An increase expression of HDAC10 is associated with autophagy induction and food restriction in rodent models or in Huh7 HCC cells treated with the mTOR inhibitor rapamycin [115]. Moreover, overexpression of HDAC10, in these cells, also promotes autophagy and decreases cell viability. High HDAC10 expression is also associated with ATGs gene expression in neuroblastoma but also to poor prognosis [116]. Since CQ addition does not further increase the accumulation of LC3-II caused by HDAC10 depletion in SK-N-BE(2)-C cells, it has been suggested that HDAC10 promotes autophagy and its depletion blocks autophagic flux. Indeed, overexpression of HDAC10 protects NB cells against doxorubicin-mediated toxicity via the acetylation of HSP70. Moreover, cotreatment of SK-N-BE(2)-C cells with doxorubicin and the specific HDAC10 inhibitor bufexamac restores doxorubicin sensitivity and cell death [116].
3.8. Ambivalent Role of the SIRT Family (Class III) in Autophagy and Cell Death
In NSCLC (A549 and H1299) cell lines, resveratrol, an activator of SIRT1, increased apoptosis and autophagy (increase of BECLIN-1 and LC3B-II, decrease of SQSTM1/P62, P-MTOR, and P-AKT) [117]. Interestingly, inhibition of SIRT1, using nicotinamide, or blockade of autophagy, using 3-MA, reduced resveratrol-mediated autophagy suggesting that autophagy was mediated by SIRT1 and was a protective event for these cells [117]. Similarly, autophagy-induced by SIRT1 is also involved in 5-FU (5-fluorouracil) resistance in colorectal cancers. Indeed, overexpression of the lncRNA H19, a sponge of miRNA, or its accumulation following 5-FU treatment in colorectal cancer cells (HCT8 and HCT116) inhibits miR-194-5p, a negative regulator of SIRT1, and thus increases SIRT1 expression and autophagy (increase of autophagosomes, LC3B-II, and decrease of SQSTM1/P62) [118]. Moreover, inhibition of autophagy using CQ, abolishes H19 dependent 5-FU resistance in these cells. SIRT1 also controls the expression of ATGs-related genes. Indeed, SIRT1 could deacetylate the RelA/p65 subunit of NFKB (nuclear factor kappa B subunit) at K310, and thus block BECN1 repression mediated by NFKB [119].
In contrast, inhibition of SIRT1 can also promote autophagy. Indeed the blockade of sirtinol-induced autophagy (accumulation of LC3B-II) using 3-MA favors cell toxicity in breast cancer MCF-7 cells [77]. Inhibition of SIRT1 (using siRNA or Ex527 inhibitor) or overexpression of hMOF restores H4K16 acetylation in HeLa cells [28]. However, blockade of autophagy (BafA1, CQ, 3-MA, or ATG7 knock-down) in these cells abrogates rapamycin + Ex524-induced cell death [28]. Inhibition of SIRT1 in endometric cancer cells (Ishikawa) or LumA (MCF-7) cells using MHY2256 also increased apoptosis and accumulated LC3B-II and eventually BECLIN-1, ATG5, ATG7, and autophagy vesicles [79,80]. Treatment of ovarian cancer cells (SKVO3) with different derivatives of a SIRT1 family inhibitors (J11-C1, 15dPGJ2, J19, or MHY2256) affected cell survival and also increased autophagy marker expression (LC3B-II, and/or BECLIN-1, and ATG13), whereas inhibition of autophagy using 3-MA strongly reduced cell death [78,80].
In leukemia/lymphoma cells, SIRT2 inhibitors NCO-90/NCO-141 also increased LC3B-II content and apoptosis but neither caspase inhibition nor BafA1 blocked NCO-90/NCO-141-induced cell death suggesting that additional mechanisms were involved [81]. The SIRT6 inhibitor UBCS039 mediated activation of autophagy via the induction of ROS accumulation which by ricochet activated the AMPK-ULK1-mTOR pathway. ROS scavengers prevented UBCS039-mediated autophagy induction. This process required the deacetylase activity of SIRT6 since the H133Y failed to induce autophagy [82].
4. HMTs and HDMs also Regulate Autophagy in Cancer Cells
Similar to histone acetylation, histone methylation, which occurs on lysine and arginine, has also been described to be involved in the control of autophagy. Methylation of histones is added by HMTs (histone methyl transferases) and removed by HDMs (histone demethylases). Some specific methylations are associated with active transcription (e.g., H3K4me2/3) but others inhibit gene expression (e.g., H3K27me3). These marks are recognized by epigenetic readers which contribute to modulate gene expression. The effects of the specific inhibition of HMTs or HDMs by epidrugs are summarized in Table 2.
4.1. G9a Represses Autophagy Genes Expression in Cancer Cells
The histone methyl transferase G9a has been shown to normally repress LC3B, WIPI, and DPTOR expression by adding the repressive H3K9me2/3 mark on their promoters but this mark has also been shown to be removed following starvation [120]. Chemical inhibition, or the use of a specific siRNA targeting G9a, led to the induction of autophagy in oral squamous cell carcinoma cells [41,120] or in bone osteosarcoma cells [35]. In breast cancers, G9a expression was inversely correlated to BECN1 expression and patients with high G9a levels and low BECN1 expression presented the worst prognosis [40]. In MCF-7 breast cancer cells, BECN1 silencing was correlated to the corecruitment of G9a and DNMT1 on its promoter. In contrast, chemical inhibition of G9a using the compound BIX-01294 promoted BECN1 expression by inducing the recruitment of NFKB on its promoter in a ROS-dependent manner. Moreover, mono-methylation of H3K9 by G9a was responsible for the activation of the serine-glycine biosynthetic pathway via the induction of the transcription of genes in response to serine deprivation [121]. Indeed, inactivation of G9a led to the depletion of serine and the induction of autophagy-linked cell death. In contrast, a high expression of G9a in cancer patients resulted in poor prognosis and elevated serine levels. In neuroblastoma BE(2)-C cells, BIX-01294 also induced the accumulation of autophagy vesicles and LC3B-II levels, whereas the silencing of G9a, using specific siRNA, increased both LC3B-I, LC3B-II, ATG3, ATG7, and ATG12 levels [42]. In glioma cells, BIX-01294 treatment resulted in an AKT-dependent increase of HIF-1α (hypoxia-inducible factor 1) expression, and therefore to increased PKM2 (pyruvate kinase M2) and TIGAR (TP53-induced glycolysis and apoptosis regulator) levels leading to LC3B increased expression [122]. In colorectal cancer cells HCT116, BIX-01294 promoted both an accumulation of GFP-LC3B vesicles and LC3B-II levels but also the induction of the transcription of ATG3, ATG4A, ATG9A, and LC3B [43]. In AGS gastric cancer cells, the flavonoid kaempferol induced autophagy (accumulation of LC3B-II, BECLIN-1, and ATG5 and decreased levels of SQSTM1/P62) and autophagy-related cell death linked to the decrease in G9a content and the stabilization of IRE1 [44]. Indeed, inhibition of autophagy, using 3-MA, CQ, siATG5, or siLC3B, restored cell viability in kaempferol-treated cells. ChIP (chromatin immuno-precipitation) experiments also revealed that G9a directly repressed LC3B expression by inducing the addition of the repressive mark H3K9me2 on its promoter. In contrast, transfection of siG9a restored LC3B expression in these cells [44].
4.2. Overexpression of EZH2 (Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit) in Cancer Cells Repress Autophagy at the Transcriptional Level
EZH2 has been shown to be recruited onto several autophagy-related promoters (TSC2, RHOA, DEPTOR, FKBP11, RGS16, and GPI) via the chromatin binding protein MTA2 (metastasis associated 1 family, member 2). This recruitment led to the inhibition of their expression via the addition of the repressive mark H3K27me3, and therefore to autophagy blockade [123]. On the one hand, inhibition of EZH2, using GSK343, increased LC3B-II levels and autophagy in U2OS bone osteosarcoma cells [35]. In the MDA-MB-231 breast cancer cells and A549 lung cancer cells, GSK343 treatment also increased LC3B-II levels, as well as the number of autophagy vesicles [37]. On the other hand, SQSTM1/P62 expression was decreased following GSK343 exposure, whereas 3-MA decreased LC3B-II levels and partially blocked GSK343-induced cell death in these cells. These data supported the idea that EZH2 negatively regulated autophagy flux, and therefore inhibited cell proliferation [37]. Moreover, inhibition of EZH2 by the chemical inhibitor 1o (N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-methyl-1-phenyl-1H-pyrazole-4-carboxamide) in neuroblastoma SK-N-BE cells and leukemia K562 cells decreased cell proliferation in an autophagy-dependent manner [38]. In colon cancer cells (HT-29 and HCT-15), inhibition of EZH2, using the compound UNC1999, increased autophagy flux [39]. However, autophagy was more induced when using a combined UNC1999/Gefitinib (EGFRi) treatment which also promoted the inhibition of cell growth and apoptosis. Inhibition of EZH2, using siRNA or EZH2i DZNep, in colorectal cancer cells (RKO and HCT116), also increased LC3B-II levels and the expression of the ATG-related protein AMBRA1 (autophagy and BECLIN-1 regulator 1) and favored apoptosis but inhibited cell proliferation and migration [33]. Similar results were obtained in the gastric cancer cells, in which the EZH2 inhibitor GSK126 promoted autophagy by decreasing phosphorylation of mTOR, AKT, and ULK1 leading to LC3B-II accumulation. These effects were further increased when GSK126 was combined with gefitinib and provoked cell death and tumor regression. This effect could be blocked when cells were first treated with the autophagy inhibitor 3-MA [34]. Similarly, in colorectal cancer cells (Lovo, HCT115, and DLD-1), inhibition of EZH2, using the chemical UNC1999 or GSK343 inhibitors, increased autophagy [36], but UNC1999 was still able to promote autophagy in ATG5-/- cells, cells treated with 3-MA, or cells expressing a dominant negative mutant of ULK1, suggesting that EZH2i could induce non-canonical autophagy in this model. Indeed, autophagy was blocked when DLD-1 cells were first treated with an inhibitor of transcription (actinomycin D) bringing to mind that EZH2i-mediated autophagy was mainly due to the induction of ATG gene transcription. Inhibition of LC3B expression using siRNA partially rescued cell viability in UNC1999-treated cells [36].
4.3. miRNA Inhibitors of EZH2 Promote Autophagy and Cell Death
The expression of miR92b and EZH2 were inversely correlated in breast cancers and overexpression of miR92b favored the accumulation of LC3B-II and GFP-LC3B vesicles and the decrease of P62/SQSTM1 in MCF-7 and MDA-MB-453 cells, during both basal or induced autophagy (starvation or rapamycin treatment) [124]. As expected, inhibition of EZH2 in the same models also led to LC3B-II and autophagy vesicle accumulation. Moreover, overexpression of miR29b strongly decreased cell proliferation, migration, and invasion, suggesting that EZH2 negatively controlled autophagy and inhibited its pro-cancer effects [124]. Similarly, the expression of miR101-3p was decreased in endometrial carcinoma (EC) as compared with normal adjacent tissues and miR101-3p was shown to negatively regulate EZH2 expression [125]. Moreover, both suppression of EZH2 expression and miR101-3p increased LC3B-II and BECLIN-1 levels [125]. In laryngeal carcinoma, a low expression of miR101 was also correlated to a high expression of EZH2. However, in this model, the ectopic expression of miR101 not only reduced EZH2 expression and cell proliferation but also decreased LC3B-II/LC3B-I levels and accumulated SQSTM1/P62, suggesting that miR101 could favor or inhibit autophagy depending of the cell model [126]. In glioma, a low expression of miR340 was associated with a poor prognosis, whereas overexpression of miR340 strongly decreased EZH2 expression and inhibited cell proliferation, migration, and invasion [127]. Moreover, forced miR340 expression also decreased phosphorylation of AKT and led to apoptosis, accumulation of LC3B-II, and decreased SQSTM1/P62 contents. In cholangiocarcinoma (CCA) cells (MZChA1, KBMC, and HuCCT1), the specific EZH2 miRNA, miR124, was also described to be decreased in tumors as compared with normal tissues [128]. miR124 or siEZH2 transfection promoted apoptosis and the accumulation of LC3B-II, BECLIN-1, and LC3B-positive vesicles in these cells. Cotransfection of siATG5 (or siBECN-1) with siEZH2 (or with miR124) inhibited cell death linked to the inhibition of EZH2 [128].
4.4. lncRNA Modulators of EZH2 Expression Modulate Autophagy in Cancer Cells at the Transcription and Post-Transcriptional Levels
In chondrosarcoma, both the expression of EZH2 and its positive regulator lncRNA HOTAIR were increased [129]. Inhibition of lncRNA HOTAIR reduced cell proliferation, the number of autophagy vesicles and LC3B-II levels in benefit of an activation of apoptosis. Indeed, the lncRNA HOTAIR could repress miR454-3p expression, in a DNA methylation manner, by inducing the recruitment of the DNMT1/EZH2 complex on the miR454-3p promoter. The levels of miR454-3p directly affected autophagy, since this miRNA can bind to the 3′-UTR of the ATG12 transcript, and thus induce its degradation [129]. Similarly, the levels of EZH2 were increased in lung cancers as compared with normal tissues and its levels were correlated to the ones of the lncRNA MSTO2P, (a new positive regulator of EZH2 whose mechanism is unknown), data which were coherent since lncRNA MSTO2P can positively regulate EZH2 [130]. As expected, the inactivation of lncRNA MSTO2P reduced EZH2 expression, as well as cell proliferation and, surprisingly, also autophagy markers (ATG5, LC3B-I, and LC3B-II), suggesting that EZH2 promoted autophagy in this model.
4.5. LSD1 (Lysine-Specific Demethylase-1) Negatively Regulates Autophagy at the Protein Level in Cancer Cells
An increase in expression of the histone demethylase LSD1 was frequently observed in cancers. Moreover, a high expression of LSD1 in neuroblastoma was correlated with poor prognosis [74]. Similarly, the expression of LSD1 was also increased in acute myeloid leukemia [72]. In neuroblastoma SH-SY5Y cells, inhibition of LSD1, using chemical inhibitors (TCP or SP2509), blocked LSD1 recruitment onto the SESN2 (sestrin2) promoter and induced its expression. Since SESN2 is an inhibitor of mTORC1, this led to the restoration of autophagy in these cells [74]. Similarly, inhibition of LSD1 in the U2OS bone osteosarcoma cells, using GSK-LSD1 or 2-PCPA, restored autophagy via the activation of the transcription of NURP1 and P62/SQSTM1 manner [35]. However, the inhibition of ULK1 or BECN-1 using siRNA had no effect on autophagy linked to LSD1-inhibition suggesting that the repression of autophagy mediated by LSD1 mainly occurs at the protein level [35]. In ovarian and endometrial carcinoma cells (ARK2, TOV112D), inhibition of LSD1, using siRNA or SP2509, increased ATG7, P62/SQSTM1, and LC3B-II levels and induced GFP-LC3B vesicle formation [75]. Moreover, LSD1 is known to interact with P62/SQSTM1 in the nucleus where it controls its stability in a methylation-independent manner. Inactivation of both P62/SQSTM1 and LSD1 in these cells strongly reduced tumor growth in rodent models. These results strongly supported the idea that the autophagy pathway regulated by LSD1 was independent of BECN-1 and different from the autophagy process induced by starvation. Inhibition of LSD1 in AML cells, using the JL1037 inhibitor, provoked LC3B-II and autophagosome accumulation [72]. Moreover, combination of JL1037 with CQ favored apoptosis in this cell model. In the ovarian carcinoma SKOV3 cells, inhibition of LSD1, using the S2101 inhibitor, activated autophagy by decreasing the phosphorylation of AKT, MTOR, and P70S6K and led to a decrease in P62/SQSTM1 and LC3B-II levels, as well as the number of LC3B-positive vesicles [71]. This effect was accompanied with an increase in apoptosis in these cells. Similarly, inhibition of LSD1, using the NCL1 inhibitor, in the prostate cancer LnCAP cells also promoted apoptosis and induced both LC3B-II levels and autophagosome accumulation [73]. Treatment of LnCAP-injected mice with NCL1 decreased tumor growth.
Some recent data suggested that autophagy could also be regulated by additional histone demethylases in cancer cells. Indeed, inhibition of KDM6B, using GSKJ4, JMD2, using ML324, or KDM5B, using PBIT, also increased LC3B-II levels and autophagy in U2OS bone osteosarcoma cells [35]. Similarly, in yeasts, Rph1 (KDM4 homolog) negatively regulated autophagy, in a demethylation independent manner, a process that could also be important in mammals to block autophagy in nutrient-rich conditions [131].
4.6. SKP2-Dependent Repression of CARM1(Coactivator-Associated Arginine Methyltransferase 1) Inhibit Autophagy at the Transcriptional Level in Cancer Cells
Nutriment starvation is a well-known autophagy inducer. Indeed, starvation led to AMPK-dependent phosphorylation of FOXO3a in the nucleus, and consequently to the repression of the SKP2 (S-phase kinase-associated protein 2) [132]. SKP2 expression was shown to be increased in HCC as compared with normal adjacent tissues and a high expression of SKP2 in cells was correlated to increased proliferation and migration [133]. Since SKP2 is a subunit of an E3 ubiquitin ligase regulating the stability of the nuclear methyltransferase CARM1, starvation-mediated repression of SKP2 resulted in an increase in CARM1 levels and its target, the permissive mark H3R17me2 [132,133]. The concomitant presence of this epigenetic mark with the autophagy transcription factor TFEB on ATG-related genes contributed to the activation of autophagy [132]. Indeed, among these targets, transcription of MAP1LC3B and ATG14 genes are directly controlled by this CARM1/TFEB during starvation, a process that could be also involved in cancers when cancer cells are frequently deprived.
5. Other Epigenetic Regulators Involved in the Control of Autophagy
5.1. The H2Bub1 Mark Positively Regulates Autophagy
A recent, and very elegant, paper revealed the role of the mono-ubiquitination of H2B (H2Bub1) and detailed the multi-epigenetic steps during starvation-induced autophagy [134]. Indeed, the authors showed that starvation in HEK293T cells induced a loss of expression of de novo DNMT3a and DNMT3b, but not of DNMT1, leading to the demethylation of the gene and the expression and activation of the deubiquitinase USP44. This increase in USP44 induced the degradation of H2Bub1 and the decrease in its recruitment onto ATG-related genes resulting in their activation. Interestingly, H2Bub1 acted upstream of hMOF since the inhibition of H2Bub1 provoked the inhibition of the acetylation of H4K16, a process associated to induced autophagy, but the inhibition of hMOF failed to modify the levels of H2Bub1.
5.2. BRD4 (Bromodomain and Extra-Terminal Domain) Represses Autophagy at the Transcriptional Level
In KP-4 cells, basal autophagy was associated with the repression of ATG-related genes mediated by BRD4 (bromodomain-containing protein 4) [31]. Indeed, BRD4 bound acetylated residues and, particularly, the positive mark H4K16ac processed by hMOF and, then, recruited the histone methyltransferase G9a in order to repress the expression of many different autophagy genes (BECN1, VMP1, PIK3C3, WIPI1, ATG2A, ATG9B, MAP1LC3B, SQSTM1, OPTN, MAP1LC3C, TECPR2, and SEC24D) via the addition of the repressive mark H3K9me2 on their promoters [31]. In contrast, the inhibition of BRD4, using specific siRNAs or the BET inhibitor JQ1, restored the expression of ATG-related genes. Although BET inhibitors are promising drugs for acute myeloid leukemia treatment, leukemia stem cells (LSC) appeared highly resistant, therefore, compromising the efficiency of these new protocols. Indeed, in JQ1-resistant leukemia stem cells, BECN1 expression, LC3B-II levels, and global autophagy were increased [135]. Chemical inhibition of autophagy, transfection of siRNA BECN1, or AMPK inhibition using compound C restored apoptosis in leukemia stem cells treated with JQ1 suggesting that, in these cells, autophagy acted as a pro-survival process which could be counteracted by a combined treatment [135].
6. Conclusions
Although autophagy has been considered for a long time to be mostly regulated at the post-translational level, it is now widely accepted that gene regulation is also determinant for the regulation of autophagy. Moreover, epigenetic modifications, DNA methylation, as well as post-translational histone modifications, events which are also highly involved in cancer promotion and regulation, are more and more linked to autophagy regulation (Figure 3). Indeed, HDACs appeared to repress autophagy signaling while HDACi could improve pro-survival signals mediated by autophagy, as well as autophagy-linked cell death. These data suggested that the inhibition of autophagy in combination with HDACi can be used against tumor cells still capable of apoptosis. Additionally, G9a, EZH2, and LSD1, which are key epigenetic repressors frequently overexpressed in cancer cells and associated with cancer-related phenotypes, have been recently suggested as new potential targets to restore autophagy cell death. Considering the importance of the autophagy-epigenetics links in cancer, it is more than likely that new specific treatments combining autophagy and epigenetic targets will be developed in the near future in order to improve anticancer therapies.
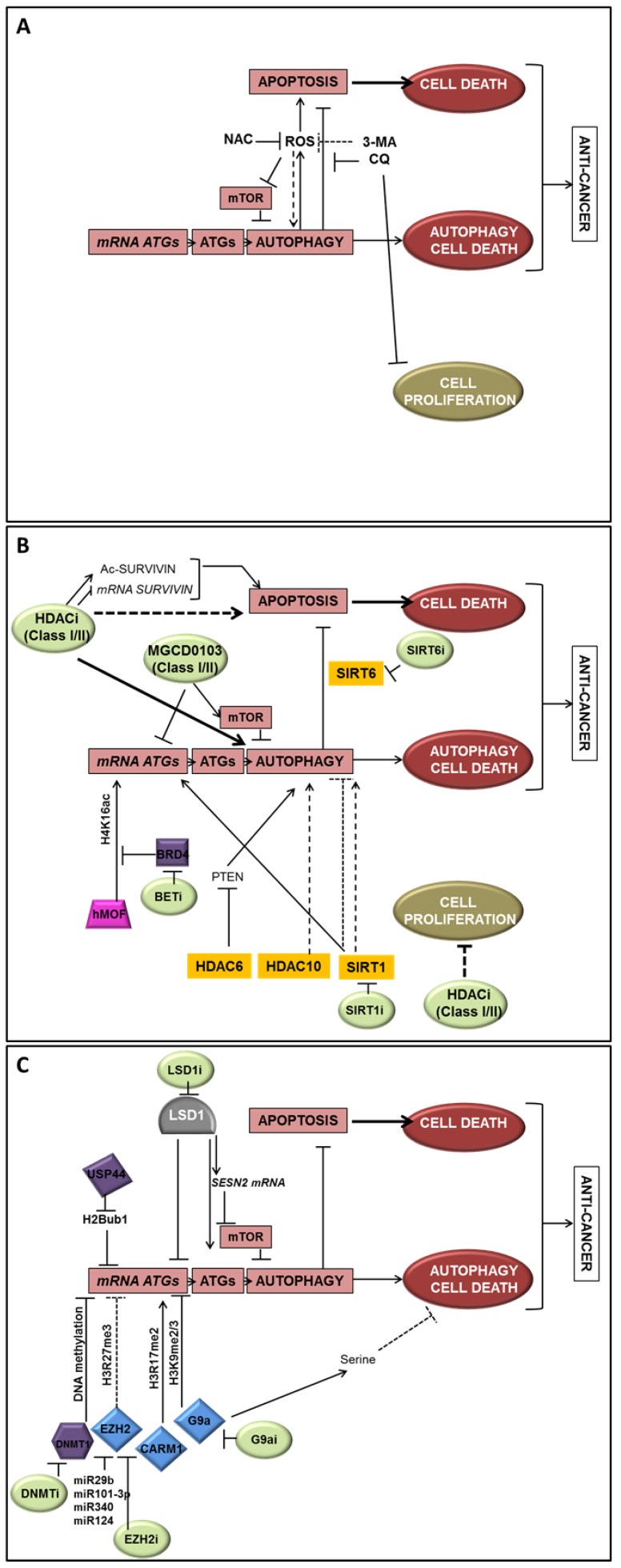
Figure 3.
Epigenetic regulation of autophagy in cancer cells. Epigenetic actors and their action on autophagy and cell death are summarized in this figure. A direct transcriptional regulation could be mediated by epigenetic modifiers to promote or inhibit ATG-related genes. An indirect regulation can also be mediated by histones deacetylases and their action on the autophagy process and ROS production. (A) Effects of autophagy inducers/inhibitors or ROS on autophagy-linked cell death or survival; (B) Effects of HDACi and acetylation/deacetylation signaling on autophagy-linked cell death or survival; (C) Effects of histone and DNA methylation regulation and histone ubiquitinylation on autophagy-linked cell death or survival. Inhibitors and miRNA specific of epigenetic modifiers are indicated when their roles have been described in the regulation of autophagy. Arrows represent a positive action on the target whereas bar-headed arrows represent an inhibition. Dotted lines are indicated when the action on the target seems indirect. (clear green: inhibitors; yellow: HDACs; blue: HMTs, pink: HATs; grey: HDMs, purple: other epigenetic proteins).
Abbreviations
ACSS2 (Acyl-CoA Synthetase-2), AKT (AKT Serine/Threonine Kinase 1), AMBRA1 (Autophagy And Beclin 1 Regulator 1), AMPK (AMP-activated protein kinase), ASC (Se-allylselenocysteine), ATG (autophagy-related), ATG4 (Autophagy-related 4), AVO (acidic vesicular organelles), 5-azadC (5-aza-deoxycytidine), BafA1 (bafilomycin-1), BET (Bromodomain and extraterminal domain) inhibitors, BMI1 (BMI1 Proto-Oncogene, Polycomb Ring Finger), BCL2L10 (Bcl-2-Like Protein 10), BNIP3 (BCL2/adenovirus E1B 19 kDa protein-interacting protein 3), CARM1 (coactivator-associated arginine methyltransferase 1), CerS6 (Ceramide Synthase 6), ChIP (chromatin immune-precipitation), CQ (chloroquine), CTSA (coding cathepsin A), DNMT (DNA methyl transferase), E2F (E2F Transcription Factor), EGFR (Epidermal Growth Factor Receptor), ER (estrogen receptor), EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit), GBA (β-glucuronidase), GUSB (β-glucosidase), HDAC (histone deacetylase), HDM (histone demethylase), HMGCR (3-Hydroxy-3-Methylglutaryl-CoA Reductase), HMT (histone methyltransferase) HR23B (RAD23 (S. Cerevisiae) Homolog B), FIH1 (Factor Inhibiting HIF1), 5-FU (5-Fluorouracil), GL1 (GABARAPL1, GABA Type A Receptor Associated Protein Like 1), H3 (Histone 3), HCC (hepatocellular carcinoma), HCQ (hydroxychloroquine), HIF-1α (hypoxia-inducible factor 1), JMD (Jumonji Domain Containing), KDM (Lysine Demethylase), LAMP1 (lysosomal membrane protein 1), LAPTM5 gene (Lysosomal-associated protein multispanning transmembrane 5), (loss of heterozygosity), LSD1 (Lysine-specific demethylase-1), 3-MA (3-methyl-adenine), MAP1LC3v1 (Microtubule Associated Protein 1 Light Chain 3 Alpha), MTOR (Mechanistic Target Of Rapamycin), NAC (N-acetyl cysteine), NFKB (Nuclear Factor Kappa B Subunit), NSCLC (non-small cell lung carcinoma), PARP (Poly(ADP-Ribose) Polymerase 1), PCAF (p300/CBP-associated factor), PCDH17 (Protocadherin 17), PKM2 (Pyruvate kinase M2), PTEN (Phosphatase And Tensin Homolog), ROS (Reactive Oxygen Species), SAHA (suberanilohydroxamic acid), SIRT (Sirtuin), SP1 (Sp1 Transcription Factor), SQSTM1 (Sequestosome 1), STAT1 (Signal Transducer And Activator Of Transcription 1), TDP-43 (TAR DNA Binding Protein), TIGAR (TP53-induced glycolysis and apoptosis regulator), TKI (Tyrosine kinase inhibitor), TNBC (triple negative BC), TSA (trichostatin A), TSS (transcriptional start site), TSG (tumor suppressor genes), USP44 (Ubiquitin Specific Peptidase 44), ULK (Unc-51 Like Autophagy Activating Kinase), UVRAG (ultraviolet irradiation resistance-associated), VPA (valproic acid).
Author Contributions
P.P., M.G. and E.H. wrote the manuscript. C.G. and J.-P.F. edited the manuscript.
Funding
This work was supported by funding from institutional grants from INSERM, EFS and Univ. Bourgogne Franche-Comté and by the “Ligue Contre le Cancer” and the “Région Bourgogne Franche-Comté”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Behrends C., Sowa M.E., Gygi S.P., Harper J.W. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Malta C., Cinque L., Settembre C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019;7:114. doi: 10.3389/fcell.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapierre L.R., Kumsta C., Sandri M., Ballabio A., Hansen M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015;11:867–880. doi: 10.1080/15548627.2015.1034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hervouet E., Cheray M., Vallette F.M., Cartron P.F. DNA methylation and apoptosis resistance in cancer cells. Cells. 2013;2:545–573. doi: 10.3390/cells2030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puto L.A., Benner C., Hunter T. The DAXX co-repressor is directly recruited to active regulatory elements genome-wide to regulate autophagy programs in a model of human prostate cancer. Oncoscience. 2015;2:362–372. doi: 10.18632/oncoscience.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puto L.A., Reed J.C. Daxx represses RelB target promoters via DNA methyltransferase recruitment and DNA hypermethylation. Genes Dev. 2008;22:998–1010. doi: 10.1101/gad.1632208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassen D.M., Bassiouny K., El-Shenawy F., Khalil H. Epigenetics Reprogramming of Autophagy is involved in Childhood Acute Lymphatic Leukemi. Pediatr. Infect. Dis. 2017;2 doi: 10.21767/2573-0282.10045. [DOI] [Google Scholar]
- 8.Li Z., Chen B., Wu Y., Jin F., Xia Y., Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10:98. doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Li C., Wang D., Chen Q., Li C.L., Li H.J. Aberrant methylation of ATG2B, ATG4D, ATG9A and ATG9B CpG island promoter is associated with decreased mRNA expression in sporadic breast carcinoma. Gene. 2016;590:285–292. doi: 10.1016/j.gene.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Swiderek E., Kalas W., Wysokinska E., Pawlak A., Rak J., Strzadala L. The interplay between epigenetic silencing, oncogenic KRas and HIF-1 regulatory pathways in control of BNIP3 expression in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013;441:707–712. doi: 10.1016/j.bbrc.2013.10.098. [DOI] [PubMed] [Google Scholar]
- 11.Hervouet E., Claude-Taupin A., Gauthier T., Perez V., Fraichard A., Adami P., Despouy G., Monnien F., Algros M.P., Jouvenot M., et al. The autophagy GABARAPL1 gene is epigenetically regulated in breast cancer models. BMC Cancer. 2015;15:729. doi: 10.1186/s12885-015-1761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhammad J.S., Nanjo S., Ando T., Yamashita S., Maekita T., Ushijima T., Tabuchi Y., Sugiyama T. Autophagy impairment by Helicobacter pylori-induced methylation silencing of MAP1LC3Av1 promotes gastric carcinogenesis. Int. J. Cancer. 2017;140:2272–2283. doi: 10.1002/ijc.30657. [DOI] [PubMed] [Google Scholar]
- 13.Bai H., Inoue J., Kawano T., Inazawa J. A transcriptional variant of the LC3A gene is involved in autophagy and frequently inactivated in human cancers. Oncogene. 2012;31:4397–4408. doi: 10.1038/onc.2011.613. [DOI] [PubMed] [Google Scholar]
- 14.Cruzeiro G.A.V., Dos Reis M.B., Silveira V.S., Lira R.C.P., Carlotti C.G., Jr., Neder L., Oliveira R.S., Yunes J.A., Brandalise S.R., Aguiar S., et al. HIF1A is Overexpressed in Medulloblastoma and its Inhibition Reduces Proliferation and Increases EPAS1 and ATG16L1 Methylation. Curr. Cancer Drug Targets. 2018;18:287–294. doi: 10.2174/1568009617666170315162525. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Schnitzler K.L., Ma Y., Nenkov M., Theis B., Petersen I. The Clinical Influence of Autophagy-Associated Proteins on Human Lung Cancer. Dis. Markers. 2018;2018:8314963. doi: 10.1155/2018/8314963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla S., Patric I.R., Patil V., Shwetha S.D., Hegde A.S., Chandramouli B.A., Arivazhagan A., Santosh V., Somasundaram K. Methylation silencing of ULK2, an autophagy gene, is essential for astrocyte transformation and tumor growth. J. Biol. Chem. 2014;289:22306–22318. doi: 10.1074/jbc.M114.567032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao Y.P., Chen L.Y., Huang R.L., Su P.H., Chan M.W., Chang C.C., Yu M.H., Wang P.H., Yen M.S., Nephew K.P., et al. Hypomethylation signature of tumor-initiating cells predicts poor prognosis of ovarian cancer patients. Hum. Mol. Genet. 2014;23:1894–1906. doi: 10.1093/hmg/ddt583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nihira K., Miki Y., Iida S., Narumi S., Ono K., Iwabuchi E., Ise K., Mori K., Saito M., Ebina M., et al. An activation of LC3A-mediated autophagy contributes to de novo and acquired resistance to EGFR tyrosine kinase inhibitors in lung adenocarcinoma. J. Pathol. 2014;234:277–288. doi: 10.1002/path.4354. [DOI] [PubMed] [Google Scholar]
- 19.Inoue J., Misawa A., Tanaka Y., Ichinose S., Sugino Y., Hosoi H., Sugimoto T., Imoto I., Inazawa J. Lysosomal-associated protein multispanning transmembrane 5 gene (LAPTM5) is associated with spontaneous regression of neuroblastomas. PLoS ONE. 2009;4:7099. doi: 10.1371/journal.pone.0007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X., Sui X., Li L., Huang X., Rong R., Su X., Shi Q., Mo L., Shu X., Kuang Y., et al. Protocadherin 17 acts as a tumour suppressor inducing tumour cell apoptosis and autophagy, and is frequently methylated in gastric and colorectal cancers. J. Pathol. 2013;229:62–73. doi: 10.1002/path.4093. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Hu X., Kuang Y., Yan P., Li L., Li C., Tao Q., Cai X. BCLB, methylated in hepatocellular carcinoma, is a starvation stress sensor that induces apoptosis and autophagy through the AMPK-mTOR signaling cascade. Cancer Lett. 2017;395:63–71. doi: 10.1016/j.canlet.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Feng J., Zhang Y., She X., Sun Y., Fan L., Ren X., Fu H., Liu C., Li P., Zhao C., et al. Hypermethylated gene ANKDD1A is a candidate tumor suppressor that interacts with FIH1 and decreases HIF1alpha stability to inhibit cell autophagy in the glioblastoma multiforme hypoxia microenvironment. Oncogene. 2019;38:103–119. doi: 10.1038/s41388-018-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M.Y., Liao W.S., Lu Z., Bornmann W.G., Hennessey V., Washington M.N., Rosner G.L., Yu Y., Ahmed A.A., Bast R.C., Jr. Decitabine and suberoylanilide hydroxamic acid (SAHA) inhibit growth of ovarian cancer cell lines and xenografts while inducing expression of imprinted tumor suppressor genes, apoptosis, G2/M arrest, and autophagy. Cancer. 2011;117:4424–4438. doi: 10.1002/cncr.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J.C., Wang F.Z., Tsai M.L., Lo C.Y., Badmaev V., Ho C.T., Wang Y.J., Pan M.H. Se-Allylselenocysteine induces autophagy by modulating the AMPK/mTOR signaling pathway and epigenetic regulation of PCDH17 in human colorectal adenocarcinoma cells. Mol. Nutr. Food Res. 2015;59:2511–2522. doi: 10.1002/mnfr.201500373. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo M., Vernier M., Mignacca L., Lessard F., Huot G., Moiseeva O., Bourdeau V., Ferbeyre G. A CDK4/6-Dependent Epigenetic Mechanism Protects Cancer Cells from PML-induced Senescence. Cancer Res. 2016;76:3252–3264. doi: 10.1158/0008-5472.CAN-15-2347. [DOI] [PubMed] [Google Scholar]
- 26.Bourdeau V., Ferbeyre G. CDK4-CDK6 inhibitors induce autophagy-mediated degradation of DNMT1 and facilitate the senescence antitumor response. Autophagy. 2016;12:1965–1966. doi: 10.1080/15548627.2016.1214779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanaei M., Kavoosi F. Histone Deacetylases and Histone Deacetylase Inhibitors: Molecular Mechanisms of Action in Various Cancers. Adv. Biomed. Res. 2019;8:63. doi: 10.4103/abr.abr_142_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fullgrabe J., Lynch-Day M.A., Heldring N., Li W., Struijk R.B., Ma Q., Hermanson O., Rosenfeld M.G., Klionsky D.J., Joseph B. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature. 2013;500:468–471. doi: 10.1038/nature12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Yu W., Qian X., Xia Y., Zheng Y., Lee J.H., Li W., Lyu J., Rao G., Zhang X., et al. Nucleus-Translocated ACSS2 Promotes Gene Transcription for Lysosomal Biogenesis and Autophagy. Mol. Cell. 2017;66:684–697. doi: 10.1016/j.molcel.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comerford S.A., Huang Z., Du X., Wang Y., Cai L., Witkiewicz A.K., Walters H., Tantawy M.N., Fu A., Manning H.C., et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamaki J.I., Wilkinson S., Hahn M., Tasdemir N., O’Prey J., Clark W., Hedley A., Nixon C., Long J.S., New M., et al. Bromodomain Protein BRD4 Is a Transcriptional Repressor of Autophagy and Lysosomal Function. Mol. Cell. 2017;66:517–532. doi: 10.1016/j.molcel.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnekenburger M., Grandjenette C., Ghelfi J., Karius T., Foliguet B., Dicato M., Diederich M. Sustained exposure to the DNA demethylating agent, 2’-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem. Pharmacol. 2011;81:364–378. doi: 10.1016/j.bcp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Yao Y., Hu H., Yang Y., Zhou G., Shang Z., Yang X., Sun K., Zhan S., Yu Z., Li P., et al. Downregulation of Enhancer of Zeste Homolog 2 (EZH2) is essential for the Induction of Autophagy and Apoptosis in Colorectal Cancer Cells. Genes (Basel) 2016;7 doi: 10.3390/genes7100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Zhu F., Wang Q., Ding Y., Ying R., Zeng L. Inhibition of EZH2 and EGFR produces a synergistic effect on cell apoptosis by increasing autophagy in gastric cancer cells. Onco Targets Ther. 2018;11:8455–8463. doi: 10.2147/OTT.S186498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Long Q.Y., Chen L., Fan J.D., Wang Z.N., Li L.Y., Wu M., Chen X. Inhibition of H3K4 demethylation induces autophagy in cancer cell lines. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:2428–2437. doi: 10.1016/j.bbamcr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh Y.Y., Lo H.L., Yang P.M. EZH2 inhibitors transcriptionally upregulate cytotoxic autophagy and cytoprotective unfolded protein response in human colorectal cancer cells. Am. J. Cancer Res. 2016;6:1661–1680. [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T.P., Lo H.L., Wei L.S., Hsiao H.H., Yang P.M. S-Adenosyl-L-methionine-competitive inhibitors of the histone methyltransferase EZH2 induce autophagy and enhance drug sensitivity in cancer cells. Anticancer Drugs. 2015;26:139–147. doi: 10.1097/CAD.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellini P., Marrocco B., Borovika D., Polletta L., Carnevale I., Saladini S., Stazi G., Zwergel C., Trapencieris P., Ferretti E., et al. Pyrazole-based inhibitors of enhancer of zeste homologue 2 induce apoptosis and autophagy in cancer cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2017.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katona B.W., Liu Y., Ma A., Jin J., Hua X. EZH2 inhibition enhances the efficacy of an EGFR inhibitor in suppressing colon cancer cells. Cancer Biol. Ther. 2014;15:1677–1687. doi: 10.4161/15384047.2014.972776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S.E., Yi H.J., Suh N., Park Y.Y., Koh J.Y., Jeong S.Y., Cho D.H., Kim C.S., Hwang J.J. Inhibition of EHMT2/G9a epigenetically increases the transcription of Beclin-1 via an increase in ROS and activation of NF-kappaB. Oncotarget. 2016;7:39796–39808. doi: 10.18632/oncotarget.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren A., Qiu Y., Cui H., Fu G. Inhibition of H3K9 methyltransferase G9a induces autophagy and apoptosis in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2015;459:10–17. doi: 10.1016/j.bbrc.2015.01.068. [DOI] [PubMed] [Google Scholar]
- 42.Ke X.X., Zhang D., Zhu S., Xia Q., Xiang Z., Cui H. Inhibition of H3K9 Methyltransferase G9a Repressed Cell Proliferation and Induced Autophagy in Neuroblastoma Cells. PLoS ONE. 2014;9:106962. doi: 10.1371/journal.pone.0106962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J.D., Lei P.J., Zheng J.Y., Wang X., Li S., Liu H., He Y.L., Wang Z.N., Wei G., Zhang X., et al. The selective activation of p53 target genes regulated by SMYD2 in BIX-01294 induced autophagy-related cell death. PLoS ONE. 2015;10:0116782. doi: 10.1371/journal.pone.0116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim T.W., Lee S.Y., Kim M., Cheon C., Ko S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9:875. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn M.Y., Ahn S.G., Yoon J.H. Apicidin, a histone deaceylase inhibitor, induces both apoptosis and autophagy in human oral squamous carcinoma cells. Oral Oncol. 2011;47:1032–1038. doi: 10.1016/j.oraloncology.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Ahn M.Y. HDAC inhibitor apicidin suppresses murine oral squamous cell carcinoma cell growth in vitro and in vivo via inhibiting HDAC8 expression. Oncol. Lett. 2018;16:6552–6560. doi: 10.3892/ol.2018.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahn M.Y., Ahn J.W., Kim H.S., Lee J., Yoon J.H. Apicidin inhibits cell growth by downregulating IGF-1R in salivary mucoepidermoid carcinoma cells. Oncol. Rep. 2015;33:1899–1907. doi: 10.3892/or.2015.3776. [DOI] [PubMed] [Google Scholar]
- 48.Shao Y., Gao Z., Marks P.A., Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marampon F., Leoni F., Mancini A., Pietrantoni I., Codenotti S., Letizia F., Megiorni F., Porro G., Galbiati E., Pozzi P., et al. Histone deacetylase inhibitor ITF2357 (givinostat) reverts transformed phenotype and counteracts stemness in in vitro and in vivo models of human glioblastoma. J. Cancer Res. Clin. Oncol. 2018 doi: 10.1007/s00432-018-2800-8. [DOI] [PubMed] [Google Scholar]
- 50.El-Khoury V., Pierson S., Szwarcbart E., Brons N.H., Roland O., Cherrier-De Wilde S., Plawny L., Van Dyck E., Berchem G. Disruption of autophagy by the histone deacetylase inhibitor MGCD0103 and its therapeutic implication in B-cell chronic lymphocytic leukemia. Leukemia. 2014;28:1636–1646. doi: 10.1038/leu.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park J.H., Ahn M.Y., Kim T.H., Yoon S., Kang K.W., Lee J., Moon H.R., Jung J.H., Chung H.Y., Kim H.S. A new synthetic HDAC inhibitor, MHY218, induces apoptosis or autophagy-related cell death in tamoxifen-resistant MCF-7 breast cancer cells. Investig. New Drugs. 2012;30:1887–1898. doi: 10.1007/s10637-011-9752-z. [DOI] [PubMed] [Google Scholar]
- 52.Klein J.M., Henke A., Sauer M., Bessler M., Reiners K.S., Engert A., Hansen H.P., von Strandmann E.P. The histone deacetylase inhibitor LBH589 (panobinostat) modulates the crosstalk of lymphocytes with Hodgkin lymphoma cell lines. PLoS ONE. 2013;8:79502. doi: 10.1371/journal.pone.0079502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lachenmayer A., Toffanin S., Cabellos L., Alsinet C., Hoshida Y., Villanueva A., Minguez B., Tsai H.W., Ward S.C., Thung S., et al. Combination therapy for hepatocellular carcinoma: Additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol. 2012;56:1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao R., Balusu R., Fiskus W., Mudunuru U., Venkannagari S., Chauhan L., Smith J.E., Hembruff S.L., Ha K., Atadja P., et al. Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol. Cancer Ther. 2012;11:973–983. doi: 10.1158/1535-7163.MCT-11-0979. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y.L., Yang P.M., Shun C.T., Wu M.S., Weng J.R., Chen C.C. Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy. 2010;6:1057–1065. doi: 10.4161/auto.6.8.13365. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto S., Tanaka K., Sakimura R., Okada T., Nakamura T., Li Y., Takasaki M., Nakabeppu Y., Iwamoto Y. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res. 2008;28:1585–1591. [PubMed] [Google Scholar]
- 57.Gammoh N., Lam D., Puente C., Ganley I., Marks P.A., Jiang X. Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc. Natl. Acad. Sci. USA. 2012;109:6561–6565. doi: 10.1073/pnas.1204429109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goncalves R.M., Agnes J.P., Delgobo M., de Souza P.O., Thome M.P., Heimfarth L., Lenz G., Moreira J.C.F., Zanotto-Filho A. Late autophagy inhibitor chloroquine improves efficacy of the histone deacetylase inhibitor SAHA and temozolomide in gliomas. Biochem. Pharmacol. 2019;163:440–450. doi: 10.1016/j.bcp.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y.J., Won A.J., Lee J., Jung J.H., Yoon S., Lee B.M., Kim H.S. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. Int. J. Med. Sci. 2012;9:881–893. doi: 10.7150/ijms.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.Y., Kuo C.W., Tsai S.L., Cheng S.M., Chen S.H., Chan H.H., Lin C.H., Lin K.Y., Li C.F., Kanwar J.R., et al. Inhibition of HDAC3- and HDAC6-Promoted Survivin Expression Plays an Important Role in SAHA-Induced Autophagy and Viability Reduction in Breast Cancer Cells. Front. Pharmacol. 2016;7:81. doi: 10.3389/fphar.2016.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.New M., Olzscha H., Liu G., Khan O., Stimson L., McGouran J., Kerr D., Coutts A., Kessler B., Middleton M., et al. A regulatory circuit that involves HR23B and HDAC6 governs the biological response to HDAC inhibitors. Cell Death Differ. 2013;20:1306–1316. doi: 10.1038/cdd.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newbold A., Vervoort S.J., Martin B.P., Bots M., Johnstone R.W. Induction of autophagy does not alter the anti-tumor effects of HDAC inhibitors. Cell Death Dis. 2012;3:387. doi: 10.1038/cddis.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu H.W., Yeh Y.L., Wang Y.C., Huang W.J., Chen Y.A., Chiou Y.S., Ho S.Y., Lin P., Wang Y.J. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, enhances radiosensitivity and suppresses lung metastasis in breast cancer in vitro and in vivo. PLoS ONE. 2013;8:76340. doi: 10.1371/journal.pone.0076340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F., Wang F., Liu Y., Wang S., Li X., Huang Y., Xia Y., Cao C. Sulforaphane induces autophagy by inhibition of HDAC6-mediated PTEN activation in triple negative breast cancer cells. Life Sci. 2018;213:149–157. doi: 10.1016/j.lfs.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 65.Wang W., Lv M., Zhao X., Zhang J. Developing a Novel Indolocarbazole as Histone Deacetylases Inhibitor against Leukemia Cell Lines. J. Anal. Methods Chem. 2015;2015:675053. doi: 10.1155/2015/675053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe M., Adachi S., Matsubara H., Imai T., Yui Y., Mizushima Y., Hiraumi Y., Watanabe K., Kamitsuji Y., Toyokuni S.Y., et al. Induction of autophagy in malignant rhabdoid tumor cells by the histone deacetylase inhibitor FK228 through AIF translocation. Int. J. Cancer. 2009;124:55–67. doi: 10.1002/ijc.23897. [DOI] [PubMed] [Google Scholar]
- 67.Dong L.H., Cheng S., Zheng Z., Wang L., Shen Y., Shen Z.X., Chen S.J., Zhao W.L. Histone deacetylase inhibitor potentiated the ability of MTOR inhibitor to induce autophagic cell death in Burkitt leukemia/lymphoma. J. Hematol. Oncol. 2013;6:53. doi: 10.1186/1756-8722-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stankov M.V., El Khatib M., Kumar Thakur B., Heitmann K., Panayotova-Dimitrova D., Schoening J., Bourquin J.P., Schweitzer N., Leverkus M., Welte K., et al. Histone deacetylase inhibitors induce apoptosis in myeloid leukemia by suppressing autophagy. Leukemia. 2014;28:577–588. doi: 10.1038/leu.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji M.M., Wang L., Zhan Q., Xue W., Zhao Y., Zhao X., Xu P.P., Shen Y., Liu H., Janin A., et al. Induction of autophagy by valproic acid enhanced lymphoma cell chemosensitivity through HDAC-independent and IP3-mediated PRKAA activation. Autophagy. 2015;11:2160–2171. doi: 10.1080/15548627.2015.1082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saha S.K., Yin Y., Kim K., Yang G.M., Dayem A.A., Choi H.Y., Cho S.G. Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng S., Jin Y., Cui M., Zheng J. Lysine-Specific Demethylase 1 (LSD1) Inhibitor S2101 Induces Autophagy via the AKT/mTOR Pathway in SKOV3 Ovarian Cancer Cells. Med. Sci. Monit. 2016;22:4742–4748. doi: 10.12659/MSM.898825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S., Lu W., Li S., Liu J., Xing Y., Zhang S., Zhou J.Z., Xing H., Xu Y., Rao Q., et al. Identification of JL1037 as a novel, specific, reversible lysine-specific demethylase 1 inhibitor that induce apoptosis and autophagy of AML cells. Oncotarget. 2017;8:31901–31914. doi: 10.18632/oncotarget.16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Etani T., Suzuki T., Naiki T., Naiki-Ito A., Ando R., Iida K., Kawai N., Tozawa K., Miyata N., Kohri K., et al. NCL1, a highly selective lysine-specific demethylase 1 inhibitor, suppresses prostate cancer without adverse effect. Oncotarget. 2015;6:2865–2878. doi: 10.18632/oncotarget.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ambrosio S., Sacca C.D., Amente S., Paladino S., Lania L., Majello B. Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway. Oncogene. 2017;36:6701–6711. doi: 10.1038/onc.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao A., Lin C.Y., Chao A.N., Tsai C.L., Chen M.Y., Lee L.Y., Chang T.C., Wang T.H., Lai C.H., Wang H.S. Lysine-specific demethylase 1 (LSD1) destabilizes p62 and inhibits autophagy in gynecologic malignancies. Oncotarget. 2017;8:74434–74450. doi: 10.18632/oncotarget.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei Y., Han T., Wang R., Wei J., Peng K., Lin Q., Shao G. LSD1 negatively regulates autophagy through the mTOR signaling pathway in ovarian cancer cells. Oncol. Rep. 2018;40:425–433. doi: 10.3892/or.2018.6432. [DOI] [PubMed] [Google Scholar]
- 77.Wang J., Kim T.H., Ahn M.Y., Lee J., Jung J.H., Choi W.S., Lee B.M., Yoon K.S., Yoon S., Kim H.S. Sirtinol, a class III HDAC inhibitor, induces apoptotic and autophagic cell death in MCF-7 human breast cancer cells. Int. J. Oncol. 2012;41:1101–1109. doi: 10.3892/ijo.2012.1534. [DOI] [PubMed] [Google Scholar]
- 78.Tae I.H., Park E.Y., Dey P., Son J.Y., Lee S.Y., Jung J.H., Saloni S., Kim M.H., Kim H.S. Novel SIRT1 inhibitor 15-deoxy-Delta12,14-prostaglandin J2 and its derivatives exhibit anticancer activity through apoptotic or autophagic cell death pathways in SKOV3 cells. Int. J. Oncol. 2018;53:2518–2530. doi: 10.3892/ijo.2018.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De U., Son J.Y., Sachan R., Park Y.J., Kang D., Yoon K., Lee B.M., Kim I.S., Moon H.R., Kim H.S. A New Synthetic Histone Deacetylase Inhibitor, MHY2256, Induces Apoptosis and Autophagy Cell Death in Endometrial Cancer Cells via p53 Acetylation. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19092743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park E.Y., Woo Y., Kim S.J., Kim D.H., Lee E.K., De U., Kim K.S., Lee J., Jung J.H., Ha K.T., et al. Anticancer Effects of a New SIRT Inhibitor, MHY2256, against Human Breast Cancer MCF-7 Cells via Regulation of MDM2-p53 Binding. Int. J. Biol. Sci. 2016;12:1555–1567. doi: 10.7150/ijbs.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gez S., Crossett B., Christopherson R.I. Differentially expressed cytosolic proteins in human leukemia and lymphoma cell lines correlate with lineages and functions. Biochim. Biophys. Acta. 2007;1774:1173–1183. doi: 10.1016/j.bbapap.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Iachettini S., Trisciuoglio D., Rotili D., Lucidi A., Salvati E., Zizza P., Di Leo L., Del Bufalo D., Ciriolo M.R., Leonetti C., et al. Pharmacological activation of SIRT6 triggers lethal autophagy in human cancer cells. Cell Death Dis. 2018;9:996. doi: 10.1038/s41419-018-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou R., Yang Y., Park S.Y., Seo Y.W., Jung S.C., Kim K.K., Kim K., Kim H. p300/CBP-associated factor promotes autophagic degradation of delta-catenin through acetylation and decreases prostate cancer tumorigenicity. Sci. Rep. 2019;9:3351. doi: 10.1038/s41598-019-40238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang F., Nguyen M., Qin F.X., Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 85.Qiao L., Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 86.Xie H.J., Noh J.H., Kim J.K., Jung K.H., Eun J.W., Bae H.J., Kim M.G., Chang Y.G., Lee J.Y., Park H., et al. HDAC1 inactivation induces mitotic defect and caspase-independent autophagic cell death in liver cancer. PLoS ONE. 2012;7:34265. doi: 10.1371/journal.pone.0034265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jo Y.K., Park N.Y., Shin J.H., Jo D.S., Bae J.E., Choi E.S., Maeng S., Jeon H.B., Roh S.A., Chang J.W., et al. Up-regulation of UVRAG by HDAC1 Inhibition Attenuates 5FU-induced Cell Death in HCT116 Colorectal Cancer Cells. Anticancer Res. 2018;38:271–277. doi: 10.21873/anticanres.12218. [DOI] [PubMed] [Google Scholar]
- 88.Carew J.S., Nawrocki S.T., Kahue C.N., Zhang H., Yang C., Chung L., Houghton J.A., Huang P., Giles F.J., Cleveland J.L. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carew J.S., Medina E.C., Esquivel J.A., 2nd, Mahalingam D., Swords R., Kelly K., Zhang H., Huang P., Mita A.C., Mita M.M., et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J. Cell Mol. Med. 2010;14:2448–2459. doi: 10.1111/j.1582-4934.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee T.G., Jeong E.H., Kim S.Y., Kim H.R., Kim C.H. The combination of irreversible EGFR TKIs and SAHA induces apoptosis and autophagy-mediated cell death to overcome acquired resistance in EGFR T790M-mutated lung cancer. Int. J. Cancer. 2015;136:2717–2729. doi: 10.1002/ijc.29320. [DOI] [PubMed] [Google Scholar]
- 91.Hui K.F., Yeung P.L., Chiang A.K. Induction of MAPK- and ROS-dependent autophagy and apoptosis in gastric carcinoma by combination of romidepsin and bortezomib. Oncotarget. 2016;7:4454–4467. doi: 10.18632/oncotarget.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Booth L., Roberts J.L., Sander C., Lee J., Kirkwood J.M., Poklepovic A., Dent P. The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo. Oncotarget. 2017;8:16367–16386. doi: 10.18632/oncotarget.14829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Booth L., Roberts J.L., Poklepovic A., Dent P. [pemetrexed + sildenafil], via autophagy-dependent HDAC downregulation, enhances the immunotherapy response of NSCLC cells. Cancer Biol. Ther. 2017;18:705–714. doi: 10.1080/15384047.2017.1362511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Booth L., Roberts J.L., Poklepovic A., Avogadri-Connors F., Cutler R.E., Lalani A.S., Dent P. HDAC inhibitors enhance neratinib activity and when combined enhance the actions of an anti-PD-1 immunomodulatory antibody in vivo. Oncotarget. 2017;8:90262–90277. doi: 10.18632/oncotarget.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Booth L., Roberts J.L., Sander C., Lalani A.S., Kirkwood J.M., Hancock J.F., Poklepovic A., Dent P. Neratinib and entinostat combine to rapidly reduce the expression of K-RAS, N-RAS, Galphaq and Galpha11 and kill uveal melanoma cells. Cancer Biol. Ther. 2019;20:700–710. doi: 10.1080/15384047.2018.1551747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan H., Li A.J., Ma S.L., Cui L.J., Wu B., Yin L., Wu M.C. Inhibition of autophagy signi fi cantly enhances combination therapy with sorafenib and HDAC inhibitors for human hepatoma cells. World J. Gastroenterol. 2014;20:4953–4962. doi: 10.3748/wjg.v20.i17.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomas S., Thurn K.T., Bicaku E., Marchion D.C., Munster P.N. Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Res. Treat. 2011;130:437–447. doi: 10.1007/s10549-011-1364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S., Li X., Wang Q., Xiu Z. Autophagy inhibitor sensitizes MCF-7 breast cancer cells to novel cyclic tetrapeptide CTS203-induced caspase-9-dependent apoptotic cell death. Neoplasma. 2015;62:220–229. doi: 10.4149/neo_2015_027. [DOI] [PubMed] [Google Scholar]
- 99.Lohitesh K., Saini H., Srivastava A., Mukherjee S., Roy A., Chowdhury R. Autophagy inhibition potentiates SAHAmediated apoptosis in glioblastoma cells by accumulation of damaged mitochondria. Oncol. Rep. 2018;39:2787–2796. doi: 10.3892/or.2018.6373. [DOI] [PubMed] [Google Scholar]
- 100.Jing B., Jin J., Xiang R., Liu M., Yang L., Tong Y., Xiao X., Lei H., Liu W., Xu H., et al. Vorinostat and quinacrine have synergistic effects in T-cell acute lymphoblastic leukemia through reactive oxygen species increase and mitophagy inhibition. Cell Death Dis. 2018;9:589. doi: 10.1038/s41419-018-0679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davood Z.A., Shamsi S., Ghaedi H., Sahand R.I., Mojtaba G., Mahdi T., Reza M., Ebrahimi M.J., Miri-Moosavi R.S., Boosaliki S., et al. Valproic acid may exerts its cytotoxic effect through rassf1a expression induction in acute myeloid leukemia. Tumour Biol. 2016;37:11001–11006. doi: 10.1007/s13277-016-4985-2. [DOI] [PubMed] [Google Scholar]
- 102.Ahn M.Y., Yoon J.H. Histone deacetylase 8 as a novel therapeutic target in oral squamous cell carcinoma. Oncol. Rep. 2017;37:540–546. doi: 10.3892/or.2016.5280. [DOI] [PubMed] [Google Scholar]
- 103.Mahalingam D., Mita M., Sarantopoulos J., Wood L., Amaravadi R.K., Davis L.E., Mita A.C., Curiel T.J., Espitia C.M., Nawrocki S.T., et al. Combined autophagy and HDAC inhibition: A phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10:1403–1414. doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin Z., Zhang Z., Jiang X., Kou X., Bao Y., Liu H., Sun F., Ling S., Qin N., Jiang L., et al. Mevastatin blockade of autolysosome maturation stimulates LBH589-induced cell death in triple-negative breast cancer cells. Oncotarget. 2017;8:17833–17848. doi: 10.18632/oncotarget.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min A., Im S.A., Kim D.K., Song S.H., Kim H.J., Lee K.H., Kim T.Y., Han S.W., Oh D.Y., O’Connor M.J., et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res. 2015;17:33. doi: 10.1186/s13058-015-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wei Q., Dong Z. HDAC4 blocks autophagy to trigger podocyte injury: Non-epigenetic action in diabetic nephropathy. Kidney Int. 2014;86:666–668. doi: 10.1038/ki.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Amodio N., Stamato M.A., Gulla A.M., Morelli E., Romeo E., Raimondi L., Pitari M.R., Ferrandino I., Misso G., Caraglia M., et al. Therapeutic Targeting of miR-29b/HDAC4 Epigenetic Loop in Multiple Myeloma. Mol. Cancer Ther. 2016;15:1364–1375. doi: 10.1158/1535-7163.MCT-15-0985. [DOI] [PubMed] [Google Scholar]
- 108.Peixoto P., Castronovo V., Matheus N., Polese C., Peulen O., Gonzalez A., Boxus M., Verdin E., Thiry M., Dequiedt F., et al. HDAC5 is required for maintenance of pericentric heterochromatin, and controls cell-cycle progression and survival of human cancer cells. Cell Death Differ. 2012;19:1239–1252. doi: 10.1038/cdd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hendrick E., Peixoto P., Blomme A., Polese C., Matheus N., Cimino J., Frere A., Mouithys-Mickalad A., Serteyn D., Bettendorff L., et al. Metabolic inhibitors accentuate the anti-tumoral effect of HDAC5 inhibition. Oncogene. 2017;36:4859–4874. doi: 10.1038/onc.2017.103. [DOI] [PubMed] [Google Scholar]
- 110.Lam H.C., Cloonan S.M., Bhashyam A.R., Haspel J.A., Singh A., Sathirapongsasuti J.F., Cervo M., Yao H., Chung A.L., Mizumura K., et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Investig. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marampon F., Megiorni F., Camero S., Crescioli C., McDowell H.P., Sferra R., Vetuschi A., Pompili S., Ventura L., De Felice F., et al. HDAC4 and HDAC6 sustain DNA double strand break repair and stem-like phenotype by promoting radioresistance in glioblastoma cells. Cancer Lett. 2017;397:1–11. doi: 10.1016/j.canlet.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 112.Lin T.W., Chen M.T., Lin L.T., Huang P.I., Lo W.L., Yang Y.P., Lu K.H., Chen Y.W., Chiou S.H., Wu C.W. TDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastoma. Oncotarget. 2017;8:56612–56625. doi: 10.18632/oncotarget.17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Booth L., Roberts J.L., Poklepovic A., Kirkwood J., Dent P. HDAC inhibitors enhance the immunotherapy response of melanoma cells. Oncotarget. 2017;8:83155–83170. doi: 10.18632/oncotarget.17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahn M.Y., Yoon J.H. Histone deacetylase 7 silencing induces apoptosis and autophagy in salivary mucoepidermoid carcinoma cells. J. Oral Pathol. Med. 2017;46:276–283. doi: 10.1111/jop.12560. [DOI] [PubMed] [Google Scholar]
- 115.Pinto G., Shtaif B., Phillip M., Gat-Yablonski G. Growth attenuation is associated with histone deacetylase 10-induced autophagy in the liver. J. Nutr. Biochem. 2016;27:171–180. doi: 10.1016/j.jnutbio.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 116.Oehme I., Linke J.P., Bock B.C., Milde T., Lodrini M., Hartenstein B., Wiegand I., Eckert C., Roth W., Kool M., et al. Histone deacetylase 10 promotes autophagy-mediated cell survival. Proc. Natl. Acad. Sci. USA. 2013;110:2592–2601. doi: 10.1073/pnas.1300113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J., Li J., Cao N., Li Z., Han J., Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777–7786. doi: 10.2147/OTT.S159095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang M., Han D., Yuan Z., Hu H., Zhao Z., Yang R., Jin Y., Zou C., Chen Y., Wang G., et al. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9:1149. doi: 10.1038/s41419-018-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nopparat C., Sinjanakhom P., Govitrapong P. Melatonin reverses H2 O2 -induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-kappaB. J. Pineal Res. 2017;63 doi: 10.1111/jpi.12407. [DOI] [PubMed] [Google Scholar]
- 120.Artal-Martinez de Narvajas A., Gomez T.S., Zhang J.S., Mann A.O., Taoda Y., Gorman J.A., Herreros-Villanueva M., Gress T.M., Ellenrieder V., Bujanda L., et al. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol. Cell Biol. 2013;33:3983–3993. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ding J., Li T., Wang X., Zhao E., Choi J.H., Yang L., Zha Y., Dong Z., Huang S., Asara J.M., et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 2013;18:896–907. doi: 10.1016/j.cmet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ahmad F., Dixit D., Joshi S.D., Sen E. G9a inhibition induced PKM2 regulates autophagic responses. Int. J. Biochem. Cell Biol. 2016;78:87–95. doi: 10.1016/j.biocel.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 123.Wei F.Z., Cao Z., Wang X., Wang H., Cai M.Y., Li T., Hattori N., Wang D., Du Y., Song B., et al. Epigenetic regulation of autophagy by the methyltransferase EZH2 through an MTOR-dependent pathway. Autophagy. 2015;11:2309–2322. doi: 10.1080/15548627.2015.1117734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu F., Sang M., Meng L., Gu L., Liu S., Li J., Geng C. miR92b promotes autophagy and suppresses viability and invasion in breast cancer by targeting EZH2. Int. J. Oncol. 2018;53:1505–1515. doi: 10.3892/ijo.2018.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang C., Liu B. miR-101-3p induces autophagy in endometrial carcinoma cells by targeting EZH2. Arch. Gynecol. Obstet. 2018;297:1539–1548. doi: 10.1007/s00404-018-4768-7. [DOI] [PubMed] [Google Scholar]
- 126.Chen L., Jia J., Zang Y., Li J., Wan B. MicroRNA-101 regulates autophagy, proliferation and apoptosis via targeting EZH2 in laryngeal squamous cell carcinoma. Neoplasma. 2019 doi: 10.4149/neo_2018_180811N611. [DOI] [PubMed] [Google Scholar]
- 127.Huang D., Qiu S., Ge R., He L., Li M., Li Y., Peng Y. miR-340 suppresses glioblastoma multiforme. Oncotarget. 2015;6:9257–9270. doi: 10.18632/oncotarget.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma J., Weng L., Wang Z., Jia Y., Liu B., Wu S., Cao Y., Sun X., Yin X., Shang M., et al. MiR-124 induces autophagy-related cell death in cholangiocarcinoma cells through direct targeting of the EZH2-STAT3 signaling axis. Exp. Cell Res. 2018;366:103–113. doi: 10.1016/j.yexcr.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 129.Bao X., Ren T., Huang Y., Sun K., Wang S., Liu K., Zheng B., Guo W. Knockdown of long non-coding RNA HOTAIR increases miR-454-3p by targeting Stat3 and Atg12 to inhibit chondrosarcoma growth. Cell Death Dis. 2017;8:2605. doi: 10.1038/cddis.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L.J., Sun G.Z., Chen Y.F. LncRNA MSTO2P promotes proliferation and autophagy of lung cancer cells by up-regulating EZH2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019;23:3375–3382. doi: 10.26355/eurrev_201904_17701. [DOI] [PubMed] [Google Scholar]
- 131.Bernard A., Jin M., Gonzalez-Rodriguez P., Fullgrabe J., Delorme-Axford E., Backues S.K., Joseph B., Klionsky D.J. Rph1/KDM4 mediates nutrient-limitation signaling that leads to the transcriptional induction of autophagy. Curr. Biol. 2015;25:546–555. doi: 10.1016/j.cub.2014.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin H.J., Kim H., Oh S., Lee J.G., Kee M., Ko H.J., Kweon M.N., Won K.J., Baek S.H. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534:553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei X., Li X., Yan W., Zhang X., Sun Y., Zhang F. SKP2 Promotes Hepatocellular Carcinoma Progression Through Nuclear AMPK-SKP2-CARM1 Signaling Transcriptionally Regulating Nutrient-Deprived Autophagy Induction. Cell Physiol. Biochem. 2018;47:2484–2497. doi: 10.1159/000491622. [DOI] [PubMed] [Google Scholar]
- 134.Chen S., Jing Y., Kang X., Yang L., Wang D.L., Zhang W., Zhang L., Chen P., Chang J.F., Yang X.M., et al. Histone H2B monoubiquitination is a critical epigenetic switch for the regulation of autophagy. Nucleic Acids Res. 2017;45:1144–1158. doi: 10.1093/nar/gkw1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jang J.E., Eom J.I., Jeung H.K., Cheong J.W., Lee J.Y., Kim J.S., Min Y.H. AMPK-ULK1-Mediated Autophagy Confers Resistance to BET Inhibitor JQ1 in Acute Myeloid Leukemia Stem Cells. Clin. Cancer Res. 2017;23:2781–2794. doi: 10.1158/1078-0432.CCR-16-1903. [DOI] [PubMed] [Google Scholar]