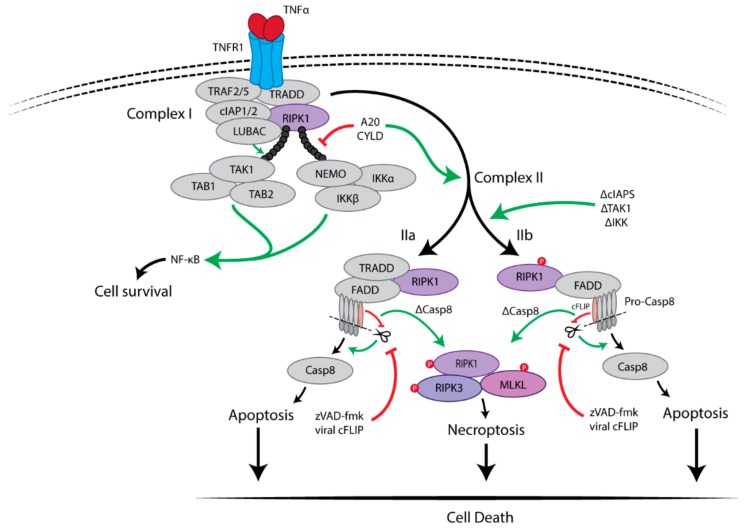
Figure 1.
TNFR1-mediated survival and cell death pathways. The binding of TNFα to TNFR1 triggers multiple signaling pathways, including NF-κB, apoptosis and necroptosis. Upon stimulation, TNFα induces the formation of a membrane-associated complex I, which consists of RIPK1, TRADD, TRAF2/5, LUBAC and cIAP1/2. Within complex I, cIAP1/2 and LUBAC induce Lys63-linked polyubiquitination of RIPK1. The polyubiquitin chain of RIPK1 serves as a scaffold for further recruitment of IKK (IKKα, IKKβ and NEMO) and TAK1(TAK1, TAB1 and TAB2) complexes, eventually leading to activation of NF-κB pathway and cell survival. Deubiquitination of RIPK1 by CYLD or A20 induces the dissociation of TRADD and RIPK1 from TNFR1, which leads to the formation of either of complex IIa or complex IIb. FADD and pro-caspase-8 are recruited to TRADD and RIPK1 to form complex IIa, resulting in the activation of caspase-8 by oligomerization and cleavage. In the absence of cIAP1/2, TAK1 or IKK complex, complex IIb, which contains RIPK1, FADD and pro-caspase-8 except TRADD, is formed and then activate caspase-8. Then, caspase-8 induces apoptosis. RIPK3-dependent necroptosis is induced when caspase-8 activity is blocked, for example by cFLIP or the pan-caspase inhibitor zVAD-fmk.