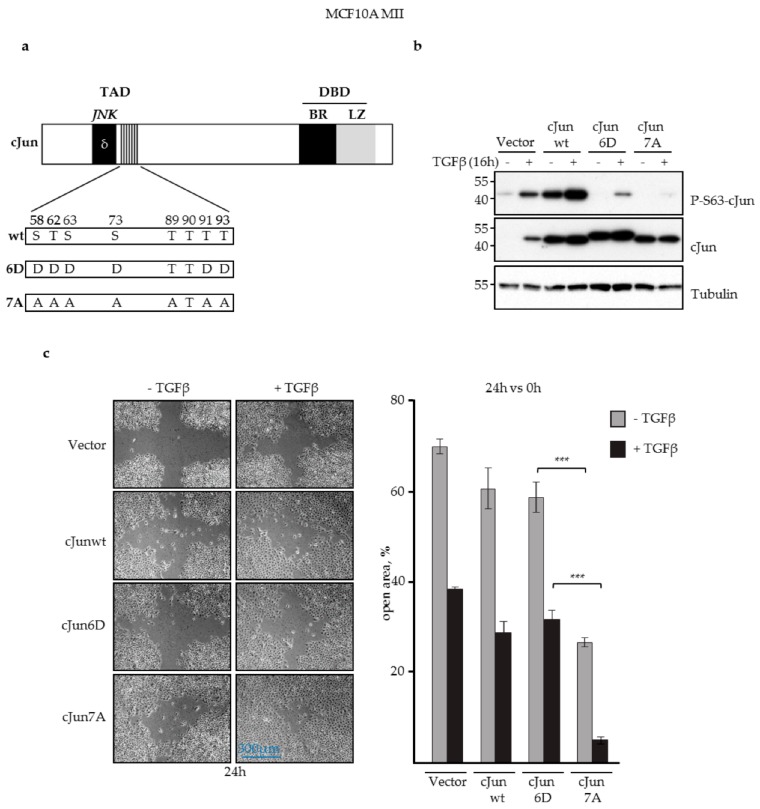
Figure 1.
JNK-dependent phosphorylation of cJun negatively affected MCF10A MII cell migration. (a) Schematic representation of cJun showing the known and potential N-terminal JNK phosphorylation sites and the changes in the 7A loss-of-function and 6D gain-of function mutants. TAD, transactivation domain; DBD, DNA-binding domain; δ, JNK docking site; BR, basic region; LZ, leucine zipper. Numbers correspond to serine/threonine phosphorylation sites in the TAD; (b) Immunoblot analysis of P-S63-cJun and cJun upon stable lentiviral overexpression of cJun wild-type (wt), cJun-6D, and cJun-7A in MCF10A MII cells cultured in the presence of EGF and treated with 5 ng/mL TGFβ for 16 h, as indicated. Tubulin was analyzed as loading control; (c) Migration (% open area) of the MCF10A MII cells stably overexpressing wt cJun as well as cJun-6D and cJun-7A mutants, in the presence or absence of TGFβ (5 ng/mL) for 24 h, as measured by wound healing assays; ***p < 0.001.