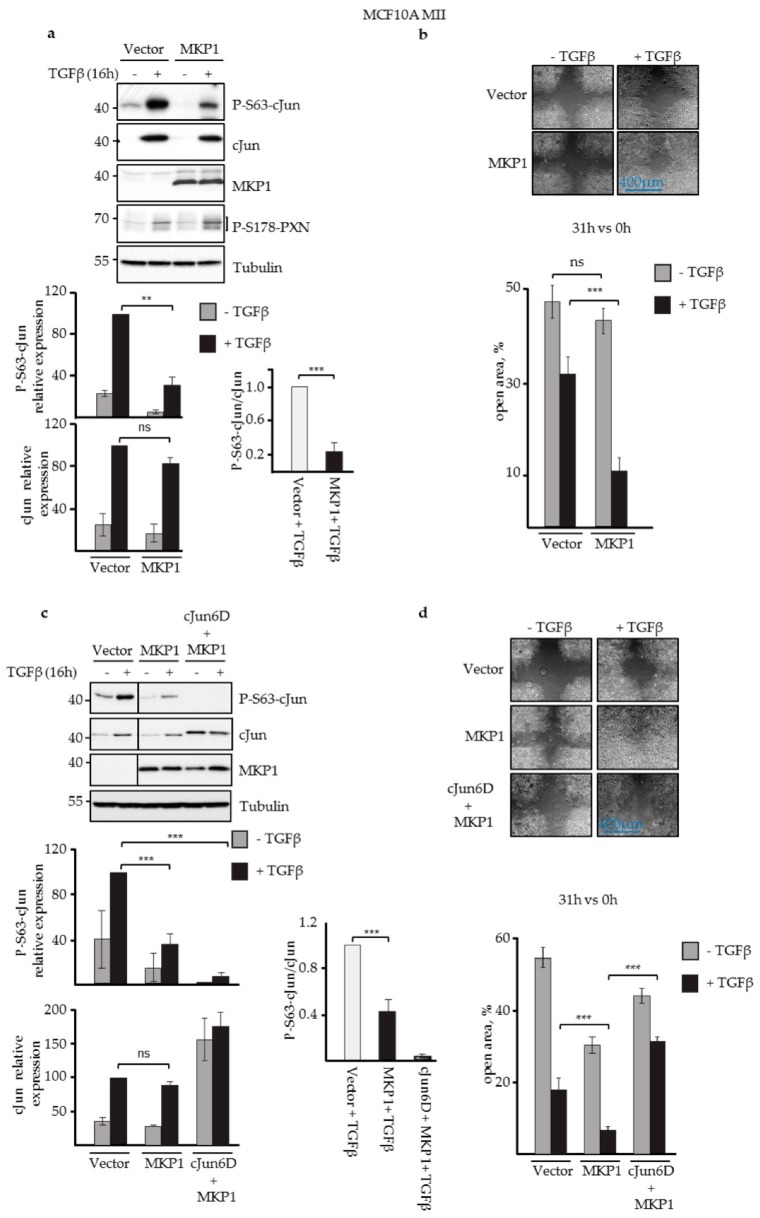
Figure 2.
JNK-specific MKP1 inhibited cJun phosphorylation and enhanced MCF10A MII cell migration. (a) Immunoblot analysis of P-S63-cJun, cJun, MKP1, and P-S178-paxillin (PXN) levels upon stable lentiviral overexpression of JNK-specific MKP1 in MCF10A MII cells and treatment with 5 ng/mL TGFβ for 16 h, as indicated. Tubulin was included as loading control; a representative experiment is shown. The average levels of P-S63-cJun and (total) cJun (quantified by densitometry and normalized to loading control) of five independent biological replicates are depicted in the graphs. For proper comparison, the TGFβ-induced P-S63-cJun and cJun levels obtained for the vector control of each experiment were set at 100. The effect of JNK-specific MKP1 on the relative ratio of the TGFβ-induced levels of P-S63-cJun and cJun is also shown, **p < 0.01, ***p < 0.001. (b) Migration (% open area) of the MCF10A MII cells stably overexpressing JNK-specific MKP1, as measured by wound healing assays in the absence or presence of TGFβ for 31 h. (c) Immunoblot analysis of MCF10A MII cells stably overexpressing cJun-6D and/or JNK-specific MKP1, in the absence and presence of TGFβ. A representative experiment is shown; the line indicates where the blot was cut; all samples were run on one gel. The average levels of P-S63-cJun, cJun, or cJun-6D (quantified by densitometry and normalized to loading control) of three independent biological replicates are depicted in graphs. For proper comparison, the TGFβ-induced P-S63-cJun and cJun levels obtained for the vector control of each experiment were set at 100. The effect of JNK-specific MKP1 on the relative ratio of the TGFβ-induced levels of P-S63-cJun and cJun or cJun-6D is also shown. (d) Migration of the MCF10A MII cells stably overexpressing cJun 6D and/or JNK-specific MKP1, as measured by wound healing assays in the absence or presence of TGFβ for 31 h.