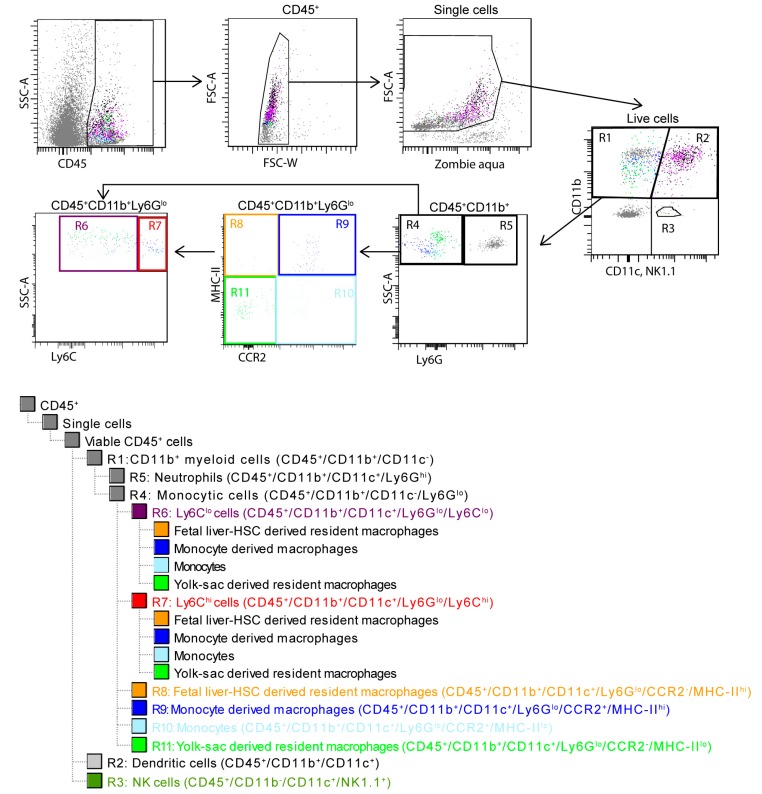
Figure 1.
Gating strategy for identifying the different immune populations in the heart. Mononuclear cells expressing CD45 were gated and doublets (FSC-W vs. FSC-A) were excluded. Dead cells were excluded by Zombie aqua. The live single CD45+ cells were then grouped into R1, CD11b+ myeloid cells (CD45+/CD11b+/CD11c−); R2, dendritic cells (CD45+/CD11b+/CD11c+); and R3, NK cells (CD45+/CD11b−/CD11c−/NK1.1+) based on their relative expression of CD11b and CD11c. R5, neutrophils (CD45+/CD11b+/CD11c-/Ly6Ghi) were then excluded from R1 based on their Ly6G expression. The remaining R4 monocytic cells were then further characterized into R6, Ly6Chi or commonly known as M1 cells (CD45+/CD11b+/CD11c−/Ly6Glo/Ly6Chi); R7, Ly6Clo or commonly known as M2 cells (CD45+/CD11b+/CD11c−/Ly6Glo/Ly6Clo) based on their Ly6C expression; and into R8, fetal liver HSC-derived resident macrophages (CD45+/CD11b+/CD11c-/Ly6Glo/CCR2−/MHC-IIhi); R9, monocyte derived macrophages (CD45+/CD11b+/CD11c-/Ly6Glo/CCR2+/MHC-IIhi); R10, monocytes (CD45+/CD11b+/CD11c−/Ly6Glo/CCR2+/MHC-IIlo); and R11, yolk sac-derived resident macrophages (CD45+/CD11b+/CD11c−/Ly6Glo/CCR2−/MHC-IIlo) based on their CCR2 and MHC-II expression. These CCR2 and MHC-II gated populations were then back gated on R6 and R7 and their relative contribution to the M1 (Ly6Chi) and M2 (Ly6Clo) cells was assessed.